RT-IC technology
Biosensor meets cytometer.
The heliXcyto instrument is the first automated biosensor for Real-Time Interaction Cytometry (RT-IC).
RT-IC can be used to characterize interactions involving antibodies, receptors, membrane targets and more where they matter:
in their native environment on cells.
Combining biophysical measurement principles with cytometry, the heliXcyto offers an automated analysis of association kinetics, dissociation kinetics, affinities and avidities. Measuring binding kinetics directly on cells preserves the complexity of the biological system:
→ Native transmembrane folding and conformational changes
→ Native target density
→ Target mobility in fluid membrane
→ Native co-interactions
A precise characterization of drug candidates in pre-clinical stages will foster higher success rates in the following expensive in vivo studies and clinical trials.
How does it work?
How single cells of any type can be capture and analyzed automatically
in tailor-made cages on heliXcyto chips.

The heliXcyto chips used in a heliXcyto instrument feature flow-permeable, bio-compatible polymer cages, which physically retain any type of cell. Trapping of adherent as well as suspension cells is possible, with no need to culture the cells on the chip surface or to use specific capture molecules for immobilization.
heliXcyto chips are available with different trap sizes to adjust to cell type and size. Furthermore, you can choose a single trap on the sensor for single cell experiments or go for five traps on the measurement spot for high signal at low expression density.
Automated workflow
How Real-Time Interaction Cytometry (RT-IC) works
on our heliXcyto instruments.
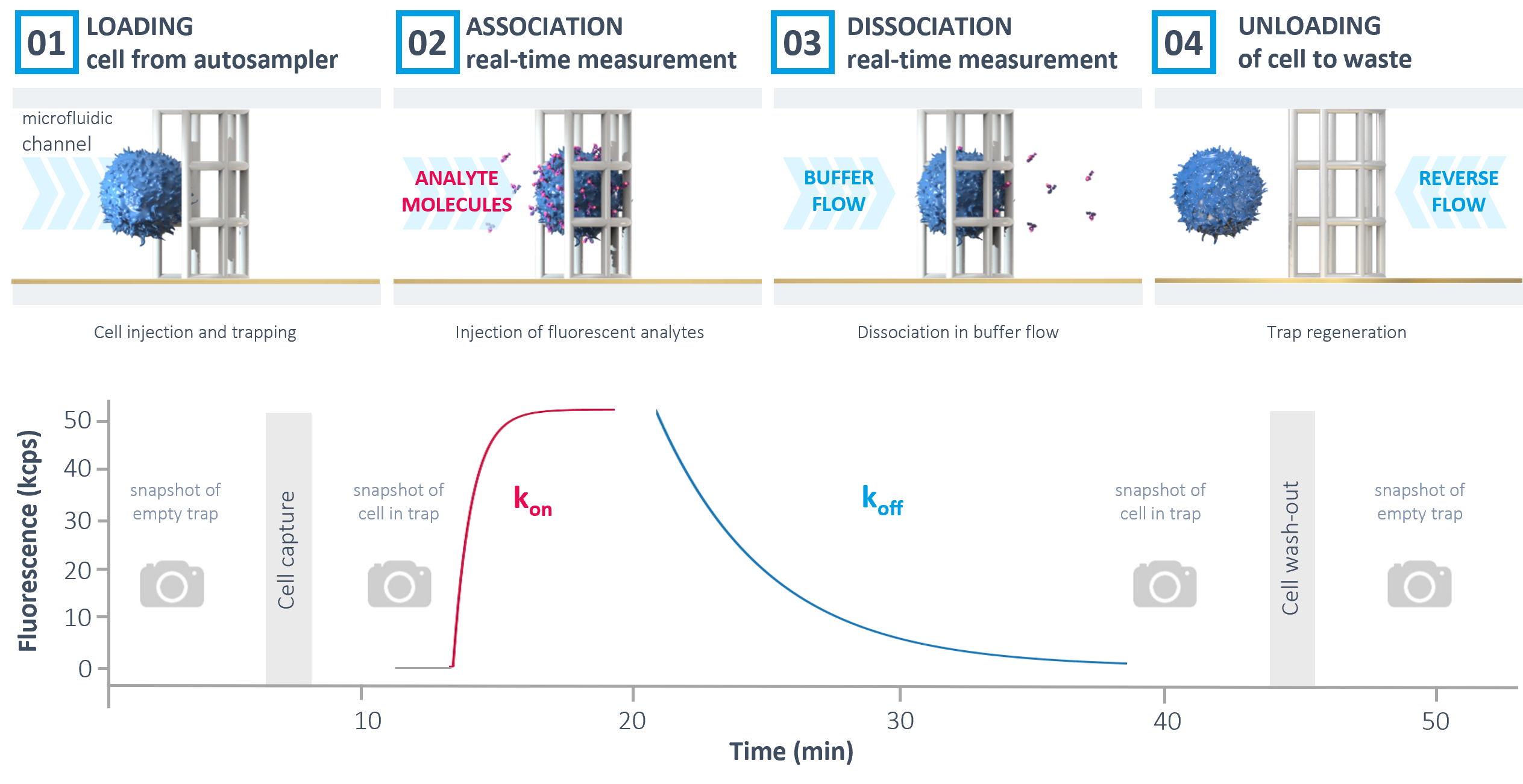
The automated workflow in the heliXcyto starts with the immobilization of the cells on the heliXcyto chip. Brightfield microscopy snapshots before and after trapping allow a visual control of the successful immobilization and quality of the cells. Afterwards, labeled analyte is automatically injected.
The association rate constant kon can be derived from the real-time increase of fluorescence on the cell. Next, dissociation kinetics (koff) can be measured by applying a constant buffer flow and monitoring the decrease in fluorescence. Finally, the cell trap is regenerated by reverse buffer flow and the chip can be reused for consecutive measurements.
FEATURES
Easy-to-use
→ Automated workflows
→ Applicable to any cell type, living or fixed
→ Compatible with complex media
→ Built-in microscope for visual inspection of cells
High sensitivity
→ Single & multiple cell analysis
→ Low membrane protein expression levels
→ Ultra-sensitive fluorescence detection
→ High performance microfluidics for resolving fast and slow kinetics
APPLICATIONS
Applications in immuno-oncology, cell therapies, and many more
Biologics development
On-cell characterization of mono- and multi-specific antibodies, and other biologics.
Adoptive cell therapies
CAR-T cells, TCR engineering, NKs, new CMC modalities.
New targets
Biophysical analysis of so far inaccessible cell surface receptors (GPCRs, …).
Contact Us
Discuss your specific needs with our specialists.