Back
Track 2 - Turbocharging Innovation in CMC: Driving Back to Rational Drug Design
(T1430-01-02) Preparation of PEG-b-PLA Nanoparticles via Inline Sonication for Controlled Release Applications
Tuesday, April 25, 2023
2:30 PM – 3:30 PM ET
- FL
Fei Liu
Evonik
- JG
James Gatewood
Scientist
Evonik
Birmingham, Alabama, United States
Poster Presenter(s)
Main Author(s)
Purpose: Previously, poly(lactide-co-glycolide) (PLG) nanoparticles prepared via an inline sonication process showed its potential as a continuous and scalable manufacturing platform (1). This poster further shows the potential of this manufacturing platform for poly(ethylene glycol)-block-polylactide (PEG-b-PLA) and poly(ethylene glycol)-block-poly(lactide-co-glycolide) (PEG-b-PLGA) nanoparticles and micelles. Nanoparticles made from PEG di-block polymers can offer various benefits over PLG nanoparticles. These benefits include improved particle formation, ability to conjugate targeting moieties to functionalized PEG, and adding stealth properties which prevent uptake by the reticuloendothelial system (2). PEG-b-PLA nanoparticles produced via inline sonication would thus present a versatile nanoencapsulation and delivery platform with a clear path from feasibility to clinical and commercial manufacturing.
Methods: Polymer solutions were prepared in dichloromethane or ethyl acetate and continuously emulsified with an aqueous polyvinyl alcohol solution using an inline sonication process. The resultant emulsion was continuously extracted in DI water to achieve a nanosuspension of dexamethasone-encapsulated nanoparticles. These nanoparticles were further processed using tangential flow filtration to remove PVA, solvent, and un-encapsulated dexamethasone. Z-average particle size and PDI were determined via dynamic light scattering analysis and dexamethasone loading was determined by HPLC method and the encapsulation efficiency calculated. Variables including polymer molecular weight PEG:PLA ratio, process solvent, and dexamethasone loading were evaluated. Polymer solutions were prepared in dichloromethane or ethyl acetate and continuously emulsified with an aqueous polyvinyl alcohol solution using an inline sonication process. The resultant emulsion was continuously extracted in DI water to achieve a nanosuspension of dexamethasone-encapsulated nanoparticles. These nanoparticles were further processed using tangential flow filtration to remove PVA, solvent, and un-encapsulated dexamethasone. Z-average particle size and PDI were determined via dynamic light scattering analysis and dexamethasone loading was determined by HPLC method and the encapsulation efficiency calculated. Variables including polymer molecular weight PEG:PLA ratio, process solvent, and dexamethasone loading were evaluated.
Results: A trend of increasing particle size with increasing PLA molecular weight was observed (Figure 3). Particles with Z-average < 200 nm and a polydispersity index < 0.3 were obtained at ideal processing and formulation conditions. Solvent selection did not appear to have a significant impact on particle size (Figure 4). Dexamethasone was observed to be successfully encapsulated at loadings between 20% and 40% with higher encapsulation efficiency observed for higher loading (Figure 5).
Conclusion: PEG-PLA nanoparticles were successfully manufactured using an inline sonication process and achieved encapsulation of a hydrophobic active pharmaceutical ingredient. The methods and materials thus described offer a scalable, versatile platform for nanoencapsulation and drug delivery.
References: (1) Operti, Maria Camilla. Pharmaceutics. 2022, 14, 276.
(2) Zhang, Keru. J. Controlled Release. 2014, 183, 77.
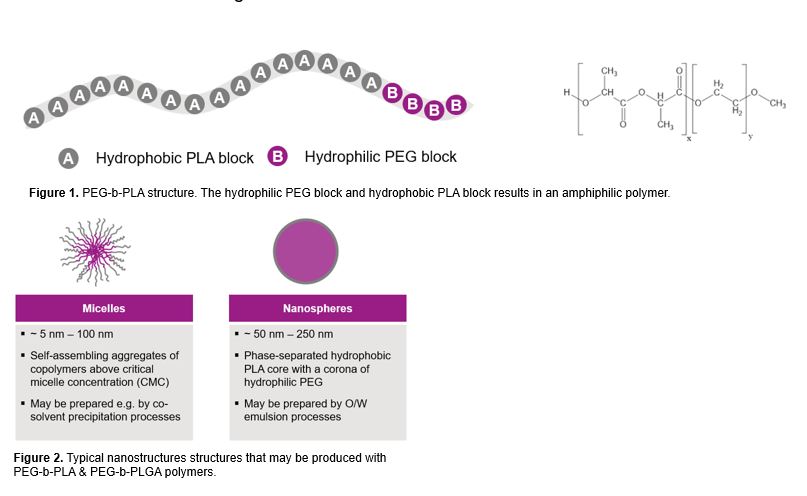
Figures 1 & 2
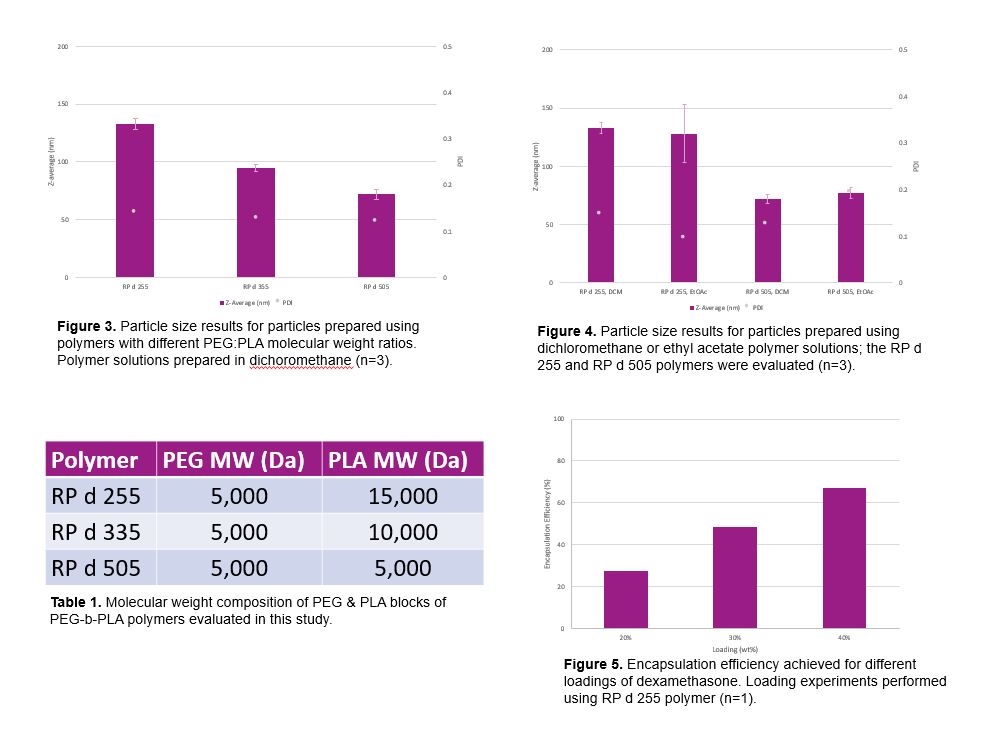
Figures 3, 4, 5, and Table 1
Methods: Polymer solutions were prepared in dichloromethane or ethyl acetate and continuously emulsified with an aqueous polyvinyl alcohol solution using an inline sonication process. The resultant emulsion was continuously extracted in DI water to achieve a nanosuspension of dexamethasone-encapsulated nanoparticles. These nanoparticles were further processed using tangential flow filtration to remove PVA, solvent, and un-encapsulated dexamethasone. Z-average particle size and PDI were determined via dynamic light scattering analysis and dexamethasone loading was determined by HPLC method and the encapsulation efficiency calculated. Variables including polymer molecular weight PEG:PLA ratio, process solvent, and dexamethasone loading were evaluated. Polymer solutions were prepared in dichloromethane or ethyl acetate and continuously emulsified with an aqueous polyvinyl alcohol solution using an inline sonication process. The resultant emulsion was continuously extracted in DI water to achieve a nanosuspension of dexamethasone-encapsulated nanoparticles. These nanoparticles were further processed using tangential flow filtration to remove PVA, solvent, and un-encapsulated dexamethasone. Z-average particle size and PDI were determined via dynamic light scattering analysis and dexamethasone loading was determined by HPLC method and the encapsulation efficiency calculated. Variables including polymer molecular weight PEG:PLA ratio, process solvent, and dexamethasone loading were evaluated.
Results: A trend of increasing particle size with increasing PLA molecular weight was observed (Figure 3). Particles with Z-average < 200 nm and a polydispersity index < 0.3 were obtained at ideal processing and formulation conditions. Solvent selection did not appear to have a significant impact on particle size (Figure 4). Dexamethasone was observed to be successfully encapsulated at loadings between 20% and 40% with higher encapsulation efficiency observed for higher loading (Figure 5).
Conclusion: PEG-PLA nanoparticles were successfully manufactured using an inline sonication process and achieved encapsulation of a hydrophobic active pharmaceutical ingredient. The methods and materials thus described offer a scalable, versatile platform for nanoencapsulation and drug delivery.
References: (1) Operti, Maria Camilla. Pharmaceutics. 2022, 14, 276.
(2) Zhang, Keru. J. Controlled Release. 2014, 183, 77.

Figures 1 & 2

Figures 3, 4, 5, and Table 1
