Back
Purpose: Malaria is one of the life-threatening diseases caused by bites of infected mosquitoes. According to WHO, 50% of the world’s population was at the risk of malaria in 2020. However, the African region had the highest share of global malaria burden. Children, within the age group of 6 months to 5 years, are the worst affected by malaria, causing about 10% of all children’s deaths in parts of the world where malaria is endemic1. Despite fatality of malaria, it is preventable and curable where many antimalarial drugs were found to be successful in treatment and prevention of malaria especially in combination therapies. Artesunate (AS)-Amodiaquine (AQ) is amongst the Artemesinin based combination therapies (ACT) currently recommended by WHO combining short and long half-life antimalarials (AS and AQ respectively) which provides better efficacy and prophylaxis after treatment. Both AS and AQ are bitter in taste leading to incompliance among paediatric populations. Also, the chemical incompatibility of both drugs and AS poor stability are major challenges when formulating AS/AQ fixed dose combination. Thus, the aim of this work is to develop stable taste masked AS/AQ micropellets using fluidized bed coating for oral administration in young children by applying the MicroCoatTM technology to address these challenges. Also, the MicroCoatTM technology prevents particle agglomeration and facilitates the process of coating small particles.
Methods: Taste masking coatings are applied separately on AS and AQ micropellets to be mixed as a fixed dose combination product. AS and AQ (micronised) were spray layered on microcrystalline cellulose microspheres (Cellets®,100-200µm), using organic and aqueous-based formulations respectively, in modified bench top fluidized bed coater (Mini-Glatt®). The drug-loaded micropellets were coated using different ratios (80/20 &75/25) of (Ethocel)/(Methocel E5) or polyvinyl pyrrolidone (PVP K30) as pore formers at 10-30% coating level (CL). MicroCoatTM technology was applied during coating whereby magnesium stearate was added as glidant into the coating chamber via an external feeding port. The coated micropellets were tested for in vitro drug release in 500 and 900mL acetate buffer pH 5.5 using USP II apparatus with paddle speed of 50 rpm at 37°C using UV and HPLC quantification of AQ and AS respectively. The in vitro taste masking effectiveness of the coating was determined using “inverting vial” method where the micropellets were dispersed in 10mL of water and AS/AQ release was measured within 15seconds-2minutes after 6 inversions of the vial.
Results: AS and AQ were layered on Cellets®100 cores and coated with taste masking coating applying MicroCoatTM technology, with no particle agglomeration and %yield for all coating trials >95%. Drug release from 80/20 (Ethocel/Methocel E5) coated AQ pellets at 10% CL was slow, releasing only 31% AQ after 45minutes. In order to achieve faster release, the %pore former was increased from 20 to 25% keeping the same coating level (10%). However, very fast AQ release was obtained (100% in 5minutes) therefore, thicker coating was applied (15-30%) to slow down release to match target release profile of >70% drug release in 45minutes. 15% CL was not enough to slow down the release (104% AQ release in 5minutes). As the thickness of the coating increased ( >15%), it was observed that AQ release further slowed down, as 20 & 30% CL showed slower AQ release (106 &88% respectively in 45minutes) (Figure 1). Results of in vitro taste masking efficiency test for AQ coated micropellets were consistent with findings from drug release tests where less drug was released from 75/25 (Ethocel/Methocel E5) coated AQ pellets as their coating thickness increased giving 9.84%±0.55, 10.6%±2.4, 1.7%±0.15 & 0.29%±0.06 for formulations at 10, 15, 20 & 30% CL respectively after 2minutes of micropellets dispersal in water. The formulation with 30% CL was further tested by the same method at different times (15seconds to 2minutes) (Figure 2) showing very low drug release slowly increasing with dispersal time. 1minute appears to be the optimum time for this test as drug release at 1minute is not that much different from that at 30seconds. For further comparison, uncoated micropellets were also tested by “inverting vial” in vitro test and the results showed high AQ release of >77% after 2minutes. For AS, 80/20 &75/25 (Ethocel/PVP K30) coated AS micropellets at 10% CL were manufactured, but their drug release results are still not satisfactory (Figure 3) thus, further AS formulation optimisation and investigation is still ongoing.
Conclusion: MicroCoatTM technology was successful in manufacturing effectively taste masked AQ coated micropellets where the 75/25 (Ethocel/Methocel E5) coated AQ pellets at 30% CL showed very low drug release (0.1%±0.043 after 1minute) with “inverting vial” test suggesting successful masking of AQ bitter taste. Also, the formulation showed achieved immediate release where 88% AQ release was obtained after 45minutes which matches the targeted release window of the currently marketed AS/AQ fixed dose tablet. Therefore, MicroCoatTM technology can improve acceptability and adherence to AQ containing anti-malarial treatments in children under 5 years.
References: 1.Retrieved from https://www.who.int/news-room/fact-sheets/detail/malaria
Acknowledgments: The authors would like to thank Unitaid for funding Fluid Pharma to carry out this project.
.jpg)
Figure 1. Percentage of AQ released from 80/20 & 75/25 (Ethocel/Methocel E5) coated micropellets at different coating levels versus time (minutes).
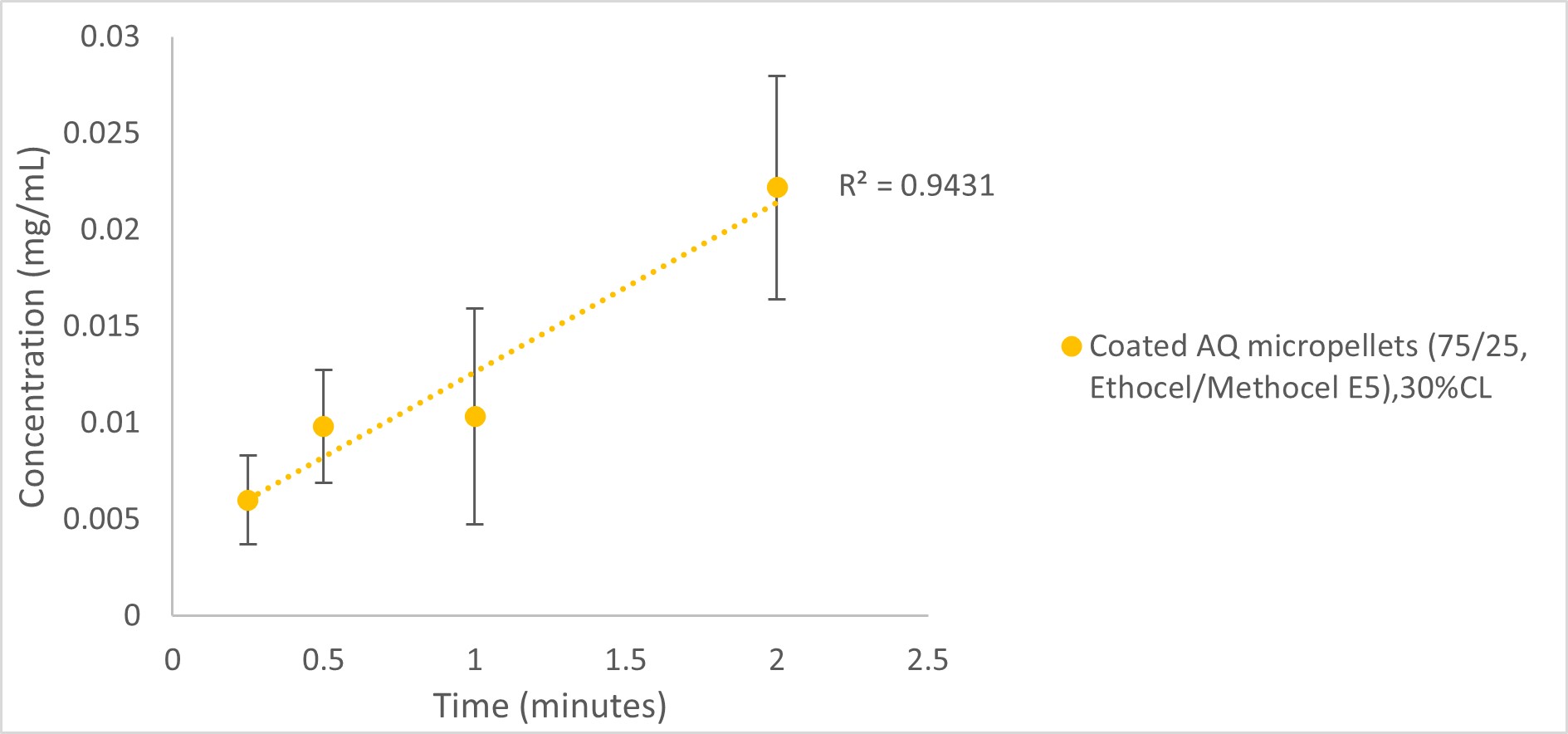
Figure 2. In vitro taste masking efficiency evaluation of 75/25 (Ethocel/Methocel E5) coated AQ micropellets (30% CL) at 15 seconds to 2 minutes.
.jpg)
Figure 3. Percentage of AS released from 80/20 & 75/25 (Ethocel/PVPK30) coated micropellets at 10% coating level versus time (minutes).
Formulation and Delivery - Chemical - Formulation
Category: Late Breaking Poster Abstract
(T1430-03-18) Application of MicroCoatTM Technology to Mask Bitter Taste of Amodiaquine and Artesunate in a Combination Antimalarial Formulation for Oral Paediatric Administration
Tuesday, October 18, 2022
2:30 PM – 3:30 PM ET
- DS
Dina Shokry, Ph.D.
University of Hertfordshire
HATFIELD, England, United Kingdom - FL
Fang Liu, Ph.D.
University of Hertfordshire
Hatfield, England, United Kingdom
Main Author(s)
Presenting Author(s)
Purpose: Malaria is one of the life-threatening diseases caused by bites of infected mosquitoes. According to WHO, 50% of the world’s population was at the risk of malaria in 2020. However, the African region had the highest share of global malaria burden. Children, within the age group of 6 months to 5 years, are the worst affected by malaria, causing about 10% of all children’s deaths in parts of the world where malaria is endemic1. Despite fatality of malaria, it is preventable and curable where many antimalarial drugs were found to be successful in treatment and prevention of malaria especially in combination therapies. Artesunate (AS)-Amodiaquine (AQ) is amongst the Artemesinin based combination therapies (ACT) currently recommended by WHO combining short and long half-life antimalarials (AS and AQ respectively) which provides better efficacy and prophylaxis after treatment. Both AS and AQ are bitter in taste leading to incompliance among paediatric populations. Also, the chemical incompatibility of both drugs and AS poor stability are major challenges when formulating AS/AQ fixed dose combination. Thus, the aim of this work is to develop stable taste masked AS/AQ micropellets using fluidized bed coating for oral administration in young children by applying the MicroCoatTM technology to address these challenges. Also, the MicroCoatTM technology prevents particle agglomeration and facilitates the process of coating small particles.
Methods: Taste masking coatings are applied separately on AS and AQ micropellets to be mixed as a fixed dose combination product. AS and AQ (micronised) were spray layered on microcrystalline cellulose microspheres (Cellets®,100-200µm), using organic and aqueous-based formulations respectively, in modified bench top fluidized bed coater (Mini-Glatt®). The drug-loaded micropellets were coated using different ratios (80/20 &75/25) of (Ethocel)/(Methocel E5) or polyvinyl pyrrolidone (PVP K30) as pore formers at 10-30% coating level (CL). MicroCoatTM technology was applied during coating whereby magnesium stearate was added as glidant into the coating chamber via an external feeding port. The coated micropellets were tested for in vitro drug release in 500 and 900mL acetate buffer pH 5.5 using USP II apparatus with paddle speed of 50 rpm at 37°C using UV and HPLC quantification of AQ and AS respectively. The in vitro taste masking effectiveness of the coating was determined using “inverting vial” method where the micropellets were dispersed in 10mL of water and AS/AQ release was measured within 15seconds-2minutes after 6 inversions of the vial.
Results: AS and AQ were layered on Cellets®100 cores and coated with taste masking coating applying MicroCoatTM technology, with no particle agglomeration and %yield for all coating trials >95%. Drug release from 80/20 (Ethocel/Methocel E5) coated AQ pellets at 10% CL was slow, releasing only 31% AQ after 45minutes. In order to achieve faster release, the %pore former was increased from 20 to 25% keeping the same coating level (10%). However, very fast AQ release was obtained (100% in 5minutes) therefore, thicker coating was applied (15-30%) to slow down release to match target release profile of >70% drug release in 45minutes. 15% CL was not enough to slow down the release (104% AQ release in 5minutes). As the thickness of the coating increased ( >15%), it was observed that AQ release further slowed down, as 20 & 30% CL showed slower AQ release (106 &88% respectively in 45minutes) (Figure 1). Results of in vitro taste masking efficiency test for AQ coated micropellets were consistent with findings from drug release tests where less drug was released from 75/25 (Ethocel/Methocel E5) coated AQ pellets as their coating thickness increased giving 9.84%±0.55, 10.6%±2.4, 1.7%±0.15 & 0.29%±0.06 for formulations at 10, 15, 20 & 30% CL respectively after 2minutes of micropellets dispersal in water. The formulation with 30% CL was further tested by the same method at different times (15seconds to 2minutes) (Figure 2) showing very low drug release slowly increasing with dispersal time. 1minute appears to be the optimum time for this test as drug release at 1minute is not that much different from that at 30seconds. For further comparison, uncoated micropellets were also tested by “inverting vial” in vitro test and the results showed high AQ release of >77% after 2minutes. For AS, 80/20 &75/25 (Ethocel/PVP K30) coated AS micropellets at 10% CL were manufactured, but their drug release results are still not satisfactory (Figure 3) thus, further AS formulation optimisation and investigation is still ongoing.
Conclusion: MicroCoatTM technology was successful in manufacturing effectively taste masked AQ coated micropellets where the 75/25 (Ethocel/Methocel E5) coated AQ pellets at 30% CL showed very low drug release (0.1%±0.043 after 1minute) with “inverting vial” test suggesting successful masking of AQ bitter taste. Also, the formulation showed achieved immediate release where 88% AQ release was obtained after 45minutes which matches the targeted release window of the currently marketed AS/AQ fixed dose tablet. Therefore, MicroCoatTM technology can improve acceptability and adherence to AQ containing anti-malarial treatments in children under 5 years.
References: 1.Retrieved from https://www.who.int/news-room/fact-sheets/detail/malaria
Acknowledgments: The authors would like to thank Unitaid for funding Fluid Pharma to carry out this project.
.jpg)
Figure 1. Percentage of AQ released from 80/20 & 75/25 (Ethocel/Methocel E5) coated micropellets at different coating levels versus time (minutes).

Figure 2. In vitro taste masking efficiency evaluation of 75/25 (Ethocel/Methocel E5) coated AQ micropellets (30% CL) at 15 seconds to 2 minutes.
.jpg)
Figure 3. Percentage of AS released from 80/20 & 75/25 (Ethocel/PVPK30) coated micropellets at 10% coating level versus time (minutes).
