Back
Purpose: The provision of patient-centric formulations is of upmost importance considering the ageing population and increasing prevalence of dysphagia, where swallowing is difficult. Orally disintegrating tablets (ODTs) help to overcome swallowing challenges presented by conventional tablets or capsules and allow for administration without water. Another treatment barrier for patients with dysphagia is high dosing complexity and this can be simplified by incorporating sustained release (SR) coatings into the formulation design. The SR coating process for microparticles smaller than 250 µm, which can improve swallowability and provide an appealing mouth feel without grittiness1, is incredibly challenging due to the tendency of agglomeration and poor powder flow; a MicroCoat™ technology has been demonstrated to successfully produce freely-flowing SR microparticles without agglomeration using Würster fluid bed coating. The aim of this research was to prepare orally disintegrating tablets (ODTs) comprising SR coated microparticles in order to simplify administration and further increase patient centricity.
Methods: SR Microparticle Preparation: Microcrystalline cellulose spheres (MCC) (Cellets® 100, Pharmatrans Sanaq AG) were layered with gliclazide (50 % drug loading) and SR coated using aqueous coated with ethyl cellulose (Surelease®, Colorcon Ltd.) and hypromellose (Opadry®, Colorcon Ltd.) in an 80:20 ratio using a fluid bed coater (Mini-Glatt®, Glatt GmbH). During coating, magnesium stearate (MS), sodium stearyl fumarate (SSF) (Pruv®, JRS Pharma) or silicon dioxide (SD) (Aerosil® 200, Evonik AG) were added as dry powder glidants into the coating chamber (0.1 % w/w coating batch per 15 min)2. Coating was carried out to achieve 20% solids weight gain. ODT Preparation: ODTs were prepared using a manual tablet press fitted with a 14 mm punch die by applying 6, 8 and 10kN compression forces to co-processed mannitol, MCC, carmellose and crospovidone (Granfiller-D™ 215, Daicel Corporation and Nichirin Chemical Co., Ltd) and SR microparticles (30%) with MS as lubricant (0.5%) (both w/w based on ODT weight). ODT Evaluation: ODT mass (n=20), thickness (n=10), hardness (n=10), friability (n=15) and disintegration (n=6) were measured according to USP standards. An additional disintegration method was applied using texture analysis (TA) (TA.XTplusC, Stable Micro Systems) with a flat probe and 5kg load cell3. 1mL deionized water was pipetted onto the tablet surface just before the probe contacted the tablet and the time taken for the gap height to plateau was measured (n=6). Single factor ANOVA tests were carried out to assess the significance of any observed differences. Scanning electron microscopy (SEM) was used to observe microparticle appearance both before and after compression to assess potential damage to the SR coating. In Vitro Drug Release Measurement: In vitro drug release (n=6) from SR microparticles was observed pre- and post-compression using USP II (paddle) apparatus and an automated closed loop UV dissolution system at 226nm (PG Instruments). Gliclazide concentration was measured in 900mL pH 7.4 phosphate buffer at 37±0.5°C for 15h using a 100rpm paddle speed. Release profiles were compared using the F2 (similarity factor) statistical model.
Results: The application of MicroCoat™ technology led to a coating process improvement with the addition of dry powder glidants providing yields greater than 95% compared to 92 % without glidant addition. Compression force had a significant influence (p < 0.05) on tablet thickness, hardness, friability and disintegration times using both methods. Using 8kN compression force, the influence of glidants added during SR coating were found to have significant influences on hardness and disintegration (Figure 1). Specifically, the inclusion of MS and SSF had a softening influence (p < 0.05) whereas SD had no influence on hardness (p=0.89). MS had no impact on disintegration time using the USP method (p=0.69) whereas SSF and. SD reduced disintegration time (p=0.0003 and 0.0004, respectively). Using the TA method, microparticles comprising MS were slowest to disintegrate at over 23s (p=0.002) however there was no observed difference between ODTs comprising no glidant, sodium stearyl fumarate and silicon dioxide at approximately 15s (p=0.18). Gliclazide release from ODTs prepared using 6 and 8kN compression force was equivalent compared to microparticles before compression (F2=81 and 68, respectively) however the change in drug release caused by the 10kN compression force was significant (F2=45) (Figure 2). After a 10kN compression force was applied, SR coating rupturing was observed from SEM images taken of the ODT matrix cross section (Figure 3).
Conclusion: The development of SR ODTs comprising coated microparticles has been shown to be a viable approach in the development of patient-centric formulations for patients with dysphagia. Prepared ODTs showed promising disintegration times ( < 30s) whilst allowing for sustained release and once daily dosing.
References: 1. Lopez, F.L., Bowles, A., Gul, M.O., Clapham, D., Ernest, T.B. and Tuleu, C., 2016. Effect of formulation variables on oral grittiness and preferences of multiparticulate formulations in adult volunteers. European Journal of Pharmaceutical Sciences, 92, pp.156-162.
2. Mohylyuk, V., Patel, K., Scott, N., Richardson, C., Murnane, D. and Liu, F., 2020. Wurster Fluidised Bed Coating of Microparticles: Towards Scalable Production of Oral Sustained-Release Liquid Medicines for Patients with Swallowing Difficulties. AAPS PharmSciTech, 21(1), p.3.
3. Abdelbary, G., Eouani, C., Prinderre, P., Joachim, J., Reynier, J.P. and Piccerelle, P.H., 2005. Determination of the in vitro disintegration profile of rapidly disintegrating tablets and correlation with oral disintegration. International journal of pharmaceutics, 292(1-2), pp.29-41.
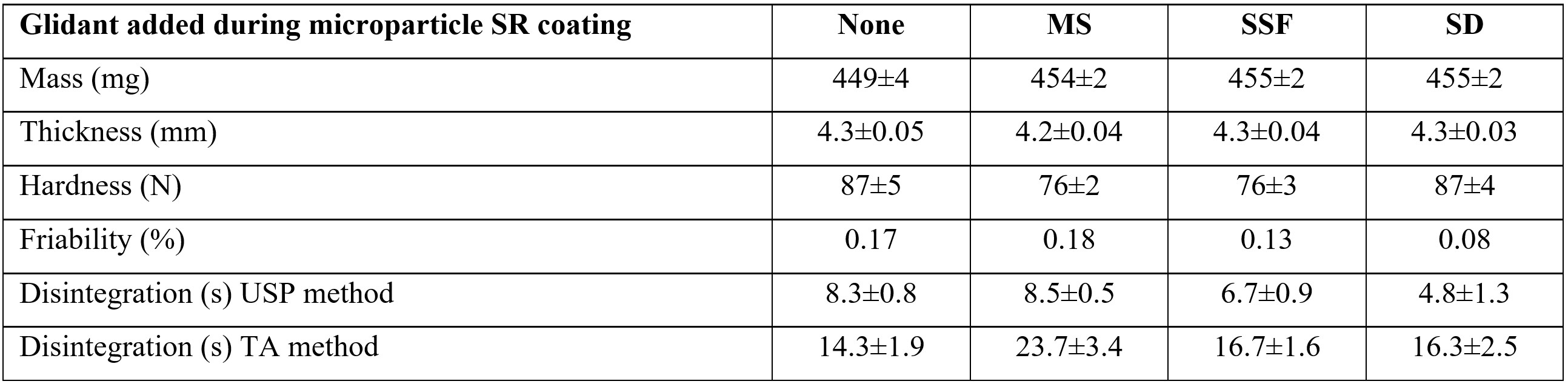
Figure 1. Physical properties for ODTs prepared using 8kN compression force showing the influence of dry powder glidant addition during SR coating of tableted microparticles
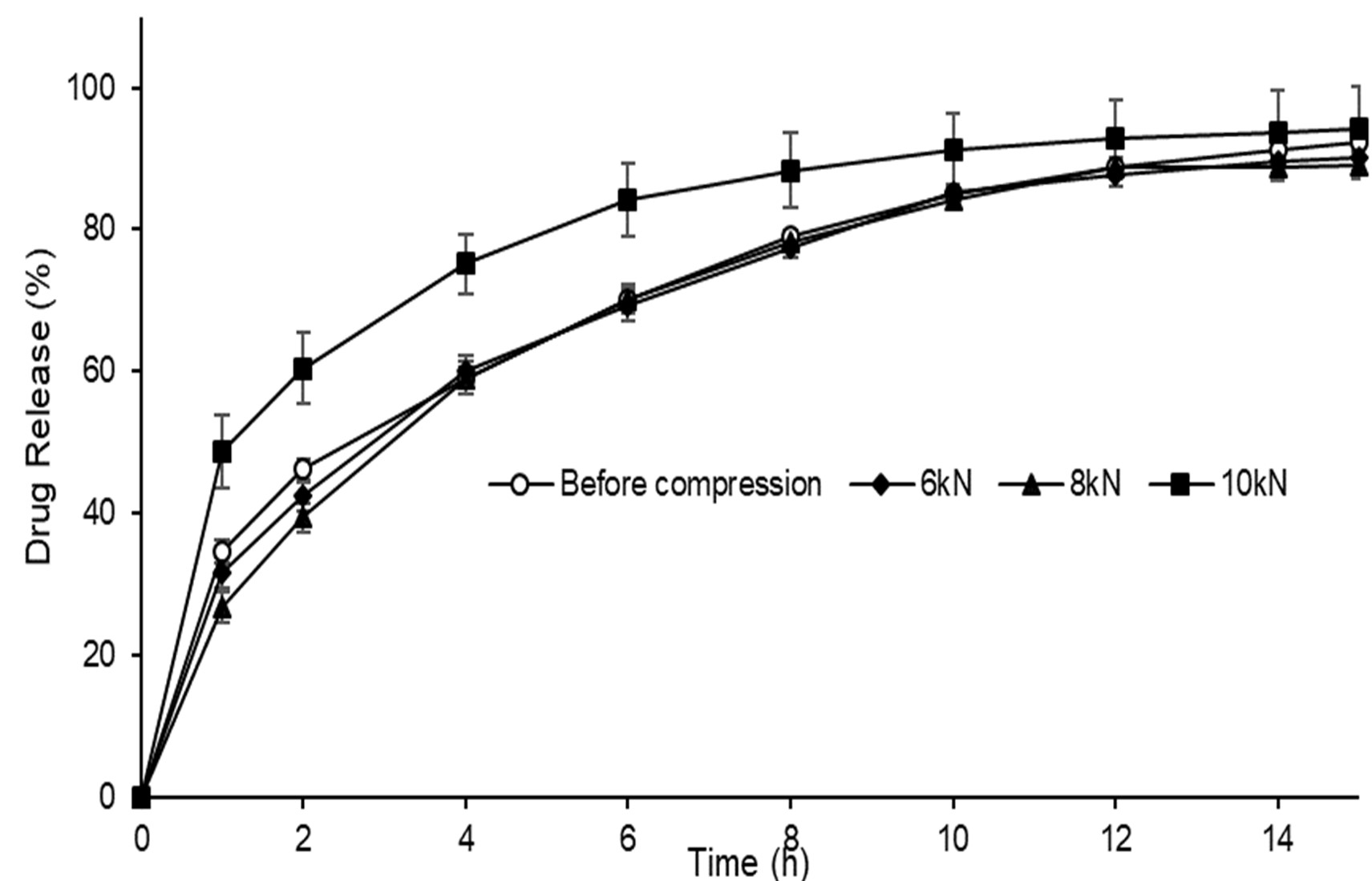
Figure 2. Gliclazide release from ODTs comprising SR coated microparticles without glidant addition prepared using various compression forces (n=6, mean±SD)

Figure 3. SEM images of prepared ODTs prepared using (a) 10kN or (b-e) 8kN compression force with microparticles coated (a-b) without or (c-e) with dry powder glidant addition during SR coating. (c) silicon dioxide (d) sodium stearyl fumarate (e) magnesium stearate
Formulation and Delivery - Chemical - Formulation
Category: Late Breaking Poster Abstract
(M1030-04-24) Sustained Release Orally Disintegrating Tablets (ODTs) Comprising Coated Microparticles to Improve Acceptability in Patients with Dysphagia
Monday, October 17, 2022
10:30 AM – 11:30 AM ET
- KP
Kavil Patel, MPharm
University of Hertfordshire
HATFIELD, England, United Kingdom - FL
Fang Liu, Ph.D.
University of Hertfordshire
Hatfield, England, United Kingdom
Main Author(s)
Presenting Author(s)
Purpose: The provision of patient-centric formulations is of upmost importance considering the ageing population and increasing prevalence of dysphagia, where swallowing is difficult. Orally disintegrating tablets (ODTs) help to overcome swallowing challenges presented by conventional tablets or capsules and allow for administration without water. Another treatment barrier for patients with dysphagia is high dosing complexity and this can be simplified by incorporating sustained release (SR) coatings into the formulation design. The SR coating process for microparticles smaller than 250 µm, which can improve swallowability and provide an appealing mouth feel without grittiness1, is incredibly challenging due to the tendency of agglomeration and poor powder flow; a MicroCoat™ technology has been demonstrated to successfully produce freely-flowing SR microparticles without agglomeration using Würster fluid bed coating. The aim of this research was to prepare orally disintegrating tablets (ODTs) comprising SR coated microparticles in order to simplify administration and further increase patient centricity.
Methods: SR Microparticle Preparation: Microcrystalline cellulose spheres (MCC) (Cellets® 100, Pharmatrans Sanaq AG) were layered with gliclazide (50 % drug loading) and SR coated using aqueous coated with ethyl cellulose (Surelease®, Colorcon Ltd.) and hypromellose (Opadry®, Colorcon Ltd.) in an 80:20 ratio using a fluid bed coater (Mini-Glatt®, Glatt GmbH). During coating, magnesium stearate (MS), sodium stearyl fumarate (SSF) (Pruv®, JRS Pharma) or silicon dioxide (SD) (Aerosil® 200, Evonik AG) were added as dry powder glidants into the coating chamber (0.1 % w/w coating batch per 15 min)2. Coating was carried out to achieve 20% solids weight gain. ODT Preparation: ODTs were prepared using a manual tablet press fitted with a 14 mm punch die by applying 6, 8 and 10kN compression forces to co-processed mannitol, MCC, carmellose and crospovidone (Granfiller-D™ 215, Daicel Corporation and Nichirin Chemical Co., Ltd) and SR microparticles (30%) with MS as lubricant (0.5%) (both w/w based on ODT weight). ODT Evaluation: ODT mass (n=20), thickness (n=10), hardness (n=10), friability (n=15) and disintegration (n=6) were measured according to USP standards. An additional disintegration method was applied using texture analysis (TA) (TA.XTplusC, Stable Micro Systems) with a flat probe and 5kg load cell3. 1mL deionized water was pipetted onto the tablet surface just before the probe contacted the tablet and the time taken for the gap height to plateau was measured (n=6). Single factor ANOVA tests were carried out to assess the significance of any observed differences. Scanning electron microscopy (SEM) was used to observe microparticle appearance both before and after compression to assess potential damage to the SR coating. In Vitro Drug Release Measurement: In vitro drug release (n=6) from SR microparticles was observed pre- and post-compression using USP II (paddle) apparatus and an automated closed loop UV dissolution system at 226nm (PG Instruments). Gliclazide concentration was measured in 900mL pH 7.4 phosphate buffer at 37±0.5°C for 15h using a 100rpm paddle speed. Release profiles were compared using the F2 (similarity factor) statistical model.
Results: The application of MicroCoat™ technology led to a coating process improvement with the addition of dry powder glidants providing yields greater than 95% compared to 92 % without glidant addition. Compression force had a significant influence (p < 0.05) on tablet thickness, hardness, friability and disintegration times using both methods. Using 8kN compression force, the influence of glidants added during SR coating were found to have significant influences on hardness and disintegration (Figure 1). Specifically, the inclusion of MS and SSF had a softening influence (p < 0.05) whereas SD had no influence on hardness (p=0.89). MS had no impact on disintegration time using the USP method (p=0.69) whereas SSF and. SD reduced disintegration time (p=0.0003 and 0.0004, respectively). Using the TA method, microparticles comprising MS were slowest to disintegrate at over 23s (p=0.002) however there was no observed difference between ODTs comprising no glidant, sodium stearyl fumarate and silicon dioxide at approximately 15s (p=0.18). Gliclazide release from ODTs prepared using 6 and 8kN compression force was equivalent compared to microparticles before compression (F2=81 and 68, respectively) however the change in drug release caused by the 10kN compression force was significant (F2=45) (Figure 2). After a 10kN compression force was applied, SR coating rupturing was observed from SEM images taken of the ODT matrix cross section (Figure 3).
Conclusion: The development of SR ODTs comprising coated microparticles has been shown to be a viable approach in the development of patient-centric formulations for patients with dysphagia. Prepared ODTs showed promising disintegration times ( < 30s) whilst allowing for sustained release and once daily dosing.
References: 1. Lopez, F.L., Bowles, A., Gul, M.O., Clapham, D., Ernest, T.B. and Tuleu, C., 2016. Effect of formulation variables on oral grittiness and preferences of multiparticulate formulations in adult volunteers. European Journal of Pharmaceutical Sciences, 92, pp.156-162.
2. Mohylyuk, V., Patel, K., Scott, N., Richardson, C., Murnane, D. and Liu, F., 2020. Wurster Fluidised Bed Coating of Microparticles: Towards Scalable Production of Oral Sustained-Release Liquid Medicines for Patients with Swallowing Difficulties. AAPS PharmSciTech, 21(1), p.3.
3. Abdelbary, G., Eouani, C., Prinderre, P., Joachim, J., Reynier, J.P. and Piccerelle, P.H., 2005. Determination of the in vitro disintegration profile of rapidly disintegrating tablets and correlation with oral disintegration. International journal of pharmaceutics, 292(1-2), pp.29-41.

Figure 1. Physical properties for ODTs prepared using 8kN compression force showing the influence of dry powder glidant addition during SR coating of tableted microparticles

Figure 2. Gliclazide release from ODTs comprising SR coated microparticles without glidant addition prepared using various compression forces (n=6, mean±SD)

Figure 3. SEM images of prepared ODTs prepared using (a) 10kN or (b-e) 8kN compression force with microparticles coated (a-b) without or (c-e) with dry powder glidant addition during SR coating. (c) silicon dioxide (d) sodium stearyl fumarate (e) magnesium stearate
