Back
Purpose: Rectal suppositories are the major dosage formulation that accounts for around 40 % of the formulations meant to be administered through the rectal route. Bioequivalence of the generic rectal suppositories is usually established by expensive and time-consuming clinical trials designed to obtain the PK endpoints. The development of an IVRT method for rectal suppositories that could establish bioequivalence of the formulations solely based on the formulation sameness, physiochemical characters, and strength may help to enhance the approval process and patient access to the generic formulations. As a part of our continuous effort to develop IVRT assays and enhance the access to the generic formulations, in the present investigation we have compared the release profile of the mesalamine fat base suppositories using the Dialysis method and Flow-through cells (USP-4 dissolution apparatus). The main objective of the present work is to develop and validate the IVRT method capable of discriminating the rectal suppositories based on their strength and critical quality attributes.
Methods: In the present investigation, for comparison, FDA-approved generic and reference (CANASA) mesalamine suppositories of 1 gram were selected. To validate the method, an In-house suppository of 600mg was prepared using the hot melt mixing method and its percent drug release was compared with the Generic and RLD formulations using the USP-4 dissolution apparatus (Sotax, MA, USA.). Regenerated cellulose membrane of mol wt. cut off 14 Kd was used as a barrier in the dialysis method. In the dialysis method, suppositories were added to 3 ml of 0.1X PBS dialyzed against 1000 ml of 0.1X PBS, pH 7.2. For the flow-through cell (USP-4) the diffusion media (0.1X PBS, pH 7.2) flow rate was maintained at 3 ml/min. The percent cumulative mesalamine release using the dialysis bag method and flow-through cells was compared and the release of the In-house formulated suppositories was performed using the flow-through cell method. In all the experiments temperature of the release medium (Perfusion media in case of flow-through cells) and the membrane was maintained at 37±0.50C. In both cases, the weight of the suppository was recorded and the amount of drug present in it was calculated. Mesalamine in each fraction was calculated using the HPLC-UV method. The discriminatory capability of both the methods was compared and validation was carried out using an In-house prepared mesalamine suppository of different strengths. The sameness of the IVRT profiles of RLD and Generic suppositories obtained from the USP-4 method was statistically tested by performing Wilcoxon signed-rank test.
Results: The suitability of each method for comparison and QC of the similar or different formulations was evaluated using the percent cumulative drug release over the period of 4 hours. The drug release profile of RLD, Generic, and In-house was compared using a flow-through cell. Percent cumulative drug release of Generic (48.588±7.499%) and RLD (49.212±8.874%) after 4 hours (Figure 1) was found to be similar and that of the In-house formulation was 44.435±2.186% (Figure 1). Whereas the cumulative drug release from RLD and Generic mesalamine suppositories performed using the Dialysis method were 38.999±12.689% and 68.689±16.635% respectively (Figure 2). The sameness of the RLD and Generic formulations was determined by performing Wilcoxon signed-rank test by using the IVRT results obtained from the USP-4 dissolution technique. The eighth and twenty-ninth ordered individual ratios are the lower and upper limits, respectively, of the 90% confidence interval for the ratio of the median in vitro release rate (slope) for Generic over the median in vitro release rate for RLD. The confidence interval is 0.84336 to 1.30193 or in percentage terms 84.336 % to 130.193 %. As this confidence interval falls within the limit of 75% to 133.33%, the products pass the Wilcoxon signed-rank test
Conclusion: This study suggests that among the tested methods, the flow-through cells were found to be the most relevant in distinguishing the mesalamine suppository. Studies conducted so far yielded promising results, thus suggesting scope for further validation for routine characterization and quality control of the rectal suppositories. Drug release profiles of the suppositories obtained using the dialysis method showed high variation with less bio-relevance when compared to flow-through cells. The flow-through cell method was found to be superior from a quality control perspective in terms of variability and reproducibility and for judging the release properties of suppositories of the same and different strengths of the formulation.
References: [1] T. J. Purohit, S. M. Hanning, and Z. Wu, “Advances in rectal drug delivery systems,” Pharm. Dev. Technol., vol. 23, no. 10, pp. 942–952, Nov. 2018, doi: 10.1080/10837450.2018.1484766.
[2] S. Hori, T. Kawada, S. Kogure, S. Yabu, K. Mori, and M. Akimoto, “Comparative release studies on suppositories using the basket, paddle, dialysis tubing and flow-through cell methods I. Acetaminophen in a lipophilic base suppository,” Pharm. Dev. Technol., vol. 22, no. 1, pp. 130–135, Jan. 2017, doi: 10.1080/10837450.2016.1230132.
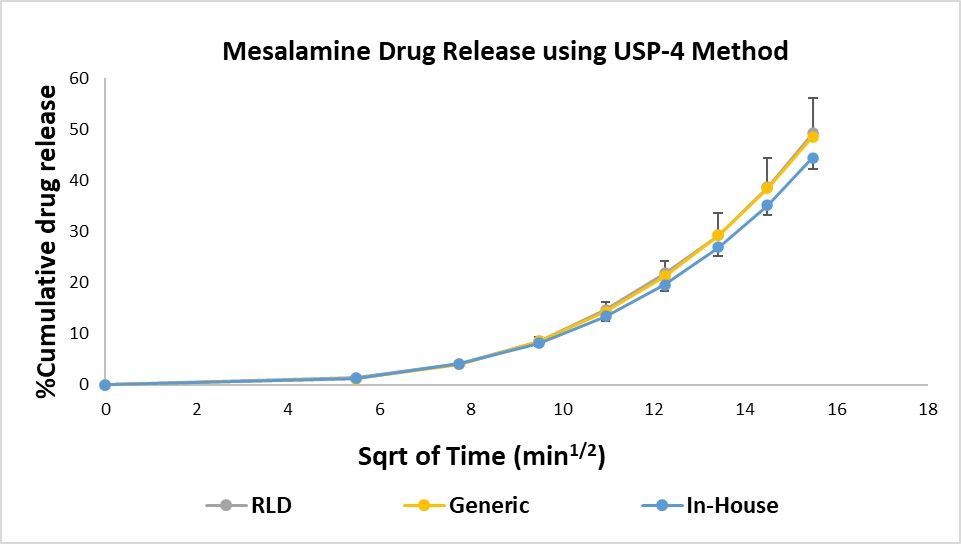
Figure 1: Drug release from different rectal suppository formulations of mesalamine using USP-4 apparatus. RLD (Canasa) and Generic suppositories release profiles (n=6; p=0.976). In-house prepared suppositories containing 600 mg of mesalamine (n=6).
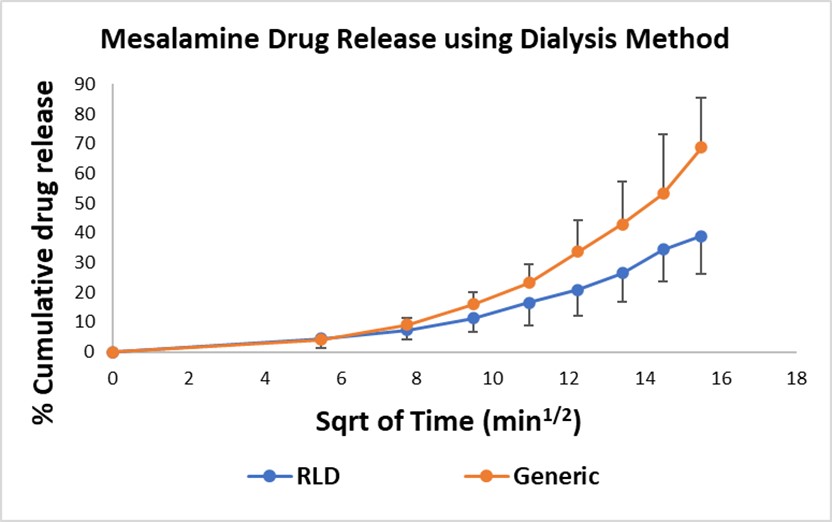
Figure 2: Drug release from rectal suppository formulations of mesalamine using Dialysis method. RLD (Canasa) and Generic mesalamine rectal suppositories (n=3; p=0.234).
Formulation and Delivery - Chemical - Formulation
Category: Poster Abstract
(W0930-03-16) Comparison of Dialysis and USP-4 Methods to Differentiate Drug Release from Mesalamine Rectal Suppositories
Wednesday, October 19, 2022
9:30 AM – 10:30 AM ET
- SP
Sushesh Srivatsa Palakurthi, MS
Texas A&M University
Kingsville, Texas, United States - SP
Sushesh Srivatsa Palakurthi, MS
Texas A&M University
Kingsville, Texas, United States
Presenting Author(s)
Main Author(s)
Purpose: Rectal suppositories are the major dosage formulation that accounts for around 40 % of the formulations meant to be administered through the rectal route. Bioequivalence of the generic rectal suppositories is usually established by expensive and time-consuming clinical trials designed to obtain the PK endpoints. The development of an IVRT method for rectal suppositories that could establish bioequivalence of the formulations solely based on the formulation sameness, physiochemical characters, and strength may help to enhance the approval process and patient access to the generic formulations. As a part of our continuous effort to develop IVRT assays and enhance the access to the generic formulations, in the present investigation we have compared the release profile of the mesalamine fat base suppositories using the Dialysis method and Flow-through cells (USP-4 dissolution apparatus). The main objective of the present work is to develop and validate the IVRT method capable of discriminating the rectal suppositories based on their strength and critical quality attributes.
Methods: In the present investigation, for comparison, FDA-approved generic and reference (CANASA) mesalamine suppositories of 1 gram were selected. To validate the method, an In-house suppository of 600mg was prepared using the hot melt mixing method and its percent drug release was compared with the Generic and RLD formulations using the USP-4 dissolution apparatus (Sotax, MA, USA.). Regenerated cellulose membrane of mol wt. cut off 14 Kd was used as a barrier in the dialysis method. In the dialysis method, suppositories were added to 3 ml of 0.1X PBS dialyzed against 1000 ml of 0.1X PBS, pH 7.2. For the flow-through cell (USP-4) the diffusion media (0.1X PBS, pH 7.2) flow rate was maintained at 3 ml/min. The percent cumulative mesalamine release using the dialysis bag method and flow-through cells was compared and the release of the In-house formulated suppositories was performed using the flow-through cell method. In all the experiments temperature of the release medium (Perfusion media in case of flow-through cells) and the membrane was maintained at 37±0.50C. In both cases, the weight of the suppository was recorded and the amount of drug present in it was calculated. Mesalamine in each fraction was calculated using the HPLC-UV method. The discriminatory capability of both the methods was compared and validation was carried out using an In-house prepared mesalamine suppository of different strengths. The sameness of the IVRT profiles of RLD and Generic suppositories obtained from the USP-4 method was statistically tested by performing Wilcoxon signed-rank test.
Results: The suitability of each method for comparison and QC of the similar or different formulations was evaluated using the percent cumulative drug release over the period of 4 hours. The drug release profile of RLD, Generic, and In-house was compared using a flow-through cell. Percent cumulative drug release of Generic (48.588±7.499%) and RLD (49.212±8.874%) after 4 hours (Figure 1) was found to be similar and that of the In-house formulation was 44.435±2.186% (Figure 1). Whereas the cumulative drug release from RLD and Generic mesalamine suppositories performed using the Dialysis method were 38.999±12.689% and 68.689±16.635% respectively (Figure 2). The sameness of the RLD and Generic formulations was determined by performing Wilcoxon signed-rank test by using the IVRT results obtained from the USP-4 dissolution technique. The eighth and twenty-ninth ordered individual ratios are the lower and upper limits, respectively, of the 90% confidence interval for the ratio of the median in vitro release rate (slope) for Generic over the median in vitro release rate for RLD. The confidence interval is 0.84336 to 1.30193 or in percentage terms 84.336 % to 130.193 %. As this confidence interval falls within the limit of 75% to 133.33%, the products pass the Wilcoxon signed-rank test
Conclusion: This study suggests that among the tested methods, the flow-through cells were found to be the most relevant in distinguishing the mesalamine suppository. Studies conducted so far yielded promising results, thus suggesting scope for further validation for routine characterization and quality control of the rectal suppositories. Drug release profiles of the suppositories obtained using the dialysis method showed high variation with less bio-relevance when compared to flow-through cells. The flow-through cell method was found to be superior from a quality control perspective in terms of variability and reproducibility and for judging the release properties of suppositories of the same and different strengths of the formulation.
References: [1] T. J. Purohit, S. M. Hanning, and Z. Wu, “Advances in rectal drug delivery systems,” Pharm. Dev. Technol., vol. 23, no. 10, pp. 942–952, Nov. 2018, doi: 10.1080/10837450.2018.1484766.
[2] S. Hori, T. Kawada, S. Kogure, S. Yabu, K. Mori, and M. Akimoto, “Comparative release studies on suppositories using the basket, paddle, dialysis tubing and flow-through cell methods I. Acetaminophen in a lipophilic base suppository,” Pharm. Dev. Technol., vol. 22, no. 1, pp. 130–135, Jan. 2017, doi: 10.1080/10837450.2016.1230132.

Figure 1: Drug release from different rectal suppository formulations of mesalamine using USP-4 apparatus. RLD (Canasa) and Generic suppositories release profiles (n=6; p=0.976). In-house prepared suppositories containing 600 mg of mesalamine (n=6).

Figure 2: Drug release from rectal suppository formulations of mesalamine using Dialysis method. RLD (Canasa) and Generic mesalamine rectal suppositories (n=3; p=0.234).
