Back
Purpose: At early stages of a pharmaceutical development program, enteric coating of hard-shell capsules is an elective strategy to achieve gastro-resistance as the time and costs to manufacture the final dosage form are significantly reduced in comparison to the development and manufacture of enteric coated tablets, granulates or pellets. When it comes to enteric coating, the formation of a continuous and homogeneous film onto the surface of the capsule shell is key to achieve gastro-resistance. In this context, the gap between the upper and lower part of the capsule is critical as the cap-body capsule junction can be accessed by gastric fluids (Fu, 2020) as the film coating could not adhere properly to the capsule surface. To prevent this, the cap-body gap can be banded with a suitable capsule banding polymer. The main objective of this study was to assess the acid-resistance and dissolution rate of banded and not-banded capsules filled with a highly water-soluble compound, at low and high filling weight, and coated with an enteric polymer at three coating levels.The purpose of the study was to evaluate: whether capsule banding was a required step in the manufacturing process to obtain gastro-resistant capsules,if the capsule filling level and the quantity of the enteric polymer applied onto the capsules had an effect on the dissolution profile of the dosage form as the coating process parameters were fixed
Methods: Niacin was used as a highly water-soluble drug model to manufacture a blend for capsule filling at 10% (w/w) drug loading. A Zanasi capsule filling machine was used to fill size 0 HPMC capsules and manufacture 10 mg (low-fill: 100 mg) and 30 mg (high-fill: 300 mg) niacin capsules. An aliquot of each capsule type was banded with an automated capsule banding machine. The coating step was conducted with a Vector LDSC machine equipped with a 2.5Lt pan (batch size ca. 1200 capsules). An aqueous Eudragit® L30 D-55 based coating suspension was prepared at 20% (w/w) solid content with talc and triethyl citrate. The target quantity of Eudragit® L30 D-55 per capsule surface area was 5.1 mg/cm2, 6.6 mg/cm2 and 9.2 mg/cm2. Following coating, the capsules were checked for appearance and a dual stage dissolution testing (USP apparatus II, acid stage: 2 hr in HCl 0.1N; basic stage: 75 min phosphate buffer pH 6.8) was conducted. The amount of niacin dissolved was determined by UV spectrophotometry at 262 nm wavelength. Not-banded capsules coated at low level were tested for dissolution also after two months storage.
Results: The powder capsule filling of 10 mg and 30 mg niacin capsules was completed successfully as well as the capsule banding step (Figure 1. The batches of banded and non-banded capsules were coated without any issues and the product temperature during spraying was kept above the film forming temperature of Eudragit® L30 D-55 (ca. 25 ºC, Evonik website, 2022). Overall, the capsule weight gain following coating was within the required range. The coated capsules presented a smooth surface (visual assessment), as the enteric film appeared homogeneously applied although, a step at the cap-body junction was observed regardless the banding (Figure 2). The banded and not-banded capsules showed gastro-resistance even at low level of coating. However, following storage, the enteric functionality was fully maintained in the not-banded capsules filled at high level whereas, the capsules filled at low level failed the test in acid conditions (Figure 3). In general, the release of niacin was completed in phosphate buffer pH 6.8 within 60 minutes and, the dissolution rate increased as the level of coating and the capsule weight was low. Within 15 minutes testing in phosphate buffer, only a limited number of capsules started to dissolve. These capsules were not-banded, low-filled and low-coated and, following two months storage, a higher number of capsules, including high-filled capsules, started to release within this timeframe.
Conclusion: Gastro-resistance and full API release in phosphate buffer pH 6.8 was achieved with both banded and not-banded capsules. In general, the enteric coat applied to the capsules guaranteed the closure of the gap at the cap-body junction. Thus, the capsule banding step can be by-passed if careful assessment of the level of coat is conducted as well as appropriate testing of the dosage form throughout storage. The coating process parameters were selected to provide suitable fluidization of the capsules in the rotating pan and obtain a visually homogeneous coat. Faster dissolution rate and reduced gastro-resistance was observed in low-filled capsules in comparison to high-filled capsules. This trend was linked to the application of the enteric film during coating and the fluidization of the capsules in the pan. Therefore, the coating process parameters have to be selected also on the basis of both the individual weight of the capsule and the batch size to be coated. This is particularly relevant if not-banded capsules are to be coated.
References: 1. Fu M, Blechar JA, Sauer A, Al-Gousous J, Langguth P. In Vitro Evaluation of Enteric-Coated HPMC Capsules-Effect of Formulation Factors on Product Performance. Pharmaceutics. 2020 Jul 23;12(8):696.
2. Evonik EUDRAGIT® Application Guidelines; Evonik Nutrition & Care GmbH: Darmstadt, Germany, 2022; Available online: https://oncare.evonik.com/ (accessed on 07 July 2022).
.jpg)
Figure 1: manufacturing process details
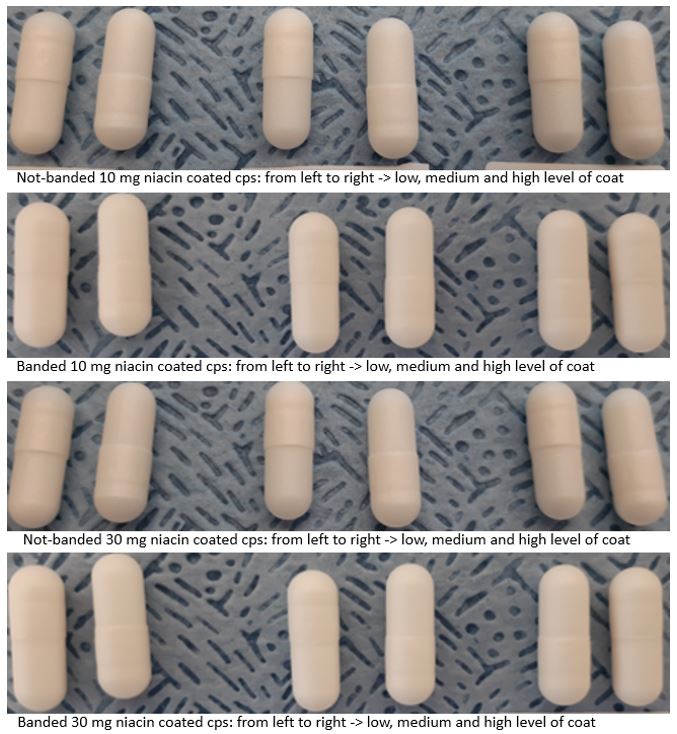
Figure 2: Coated capsules
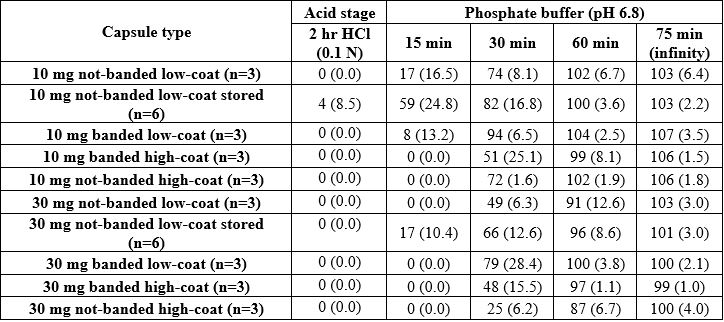
Figure 3: Dissolution testing
Formulation and Delivery - Chemical - Formulation
Category: Late Breaking Poster Abstract
(M1530-03-16) An Assessment of the Gastro-Resistance and Dissolution Rate of Banded and Not-Banded Capsules Coated with an Enteric Coating Film
Monday, October 17, 2022
3:30 PM – 4:30 PM ET
- PP
Piero Piccinni, MS
Aptuit (Verona) Srl, an Evotec Company
Verona, Veneto, Italy - PP
Piero Piccinni, MS
Aptuit (Verona) Srl, an Evotec Company
Verona, Veneto, Italy
Presenting Author(s)
Main Author(s)
Purpose: At early stages of a pharmaceutical development program, enteric coating of hard-shell capsules is an elective strategy to achieve gastro-resistance as the time and costs to manufacture the final dosage form are significantly reduced in comparison to the development and manufacture of enteric coated tablets, granulates or pellets. When it comes to enteric coating, the formation of a continuous and homogeneous film onto the surface of the capsule shell is key to achieve gastro-resistance. In this context, the gap between the upper and lower part of the capsule is critical as the cap-body capsule junction can be accessed by gastric fluids (Fu, 2020) as the film coating could not adhere properly to the capsule surface. To prevent this, the cap-body gap can be banded with a suitable capsule banding polymer. The main objective of this study was to assess the acid-resistance and dissolution rate of banded and not-banded capsules filled with a highly water-soluble compound, at low and high filling weight, and coated with an enteric polymer at three coating levels.The purpose of the study was to evaluate: whether capsule banding was a required step in the manufacturing process to obtain gastro-resistant capsules,if the capsule filling level and the quantity of the enteric polymer applied onto the capsules had an effect on the dissolution profile of the dosage form as the coating process parameters were fixed
Methods: Niacin was used as a highly water-soluble drug model to manufacture a blend for capsule filling at 10% (w/w) drug loading. A Zanasi capsule filling machine was used to fill size 0 HPMC capsules and manufacture 10 mg (low-fill: 100 mg) and 30 mg (high-fill: 300 mg) niacin capsules. An aliquot of each capsule type was banded with an automated capsule banding machine. The coating step was conducted with a Vector LDSC machine equipped with a 2.5Lt pan (batch size ca. 1200 capsules). An aqueous Eudragit® L30 D-55 based coating suspension was prepared at 20% (w/w) solid content with talc and triethyl citrate. The target quantity of Eudragit® L30 D-55 per capsule surface area was 5.1 mg/cm2, 6.6 mg/cm2 and 9.2 mg/cm2. Following coating, the capsules were checked for appearance and a dual stage dissolution testing (USP apparatus II, acid stage: 2 hr in HCl 0.1N; basic stage: 75 min phosphate buffer pH 6.8) was conducted. The amount of niacin dissolved was determined by UV spectrophotometry at 262 nm wavelength. Not-banded capsules coated at low level were tested for dissolution also after two months storage.
Results: The powder capsule filling of 10 mg and 30 mg niacin capsules was completed successfully as well as the capsule banding step (Figure 1. The batches of banded and non-banded capsules were coated without any issues and the product temperature during spraying was kept above the film forming temperature of Eudragit® L30 D-55 (ca. 25 ºC, Evonik website, 2022). Overall, the capsule weight gain following coating was within the required range. The coated capsules presented a smooth surface (visual assessment), as the enteric film appeared homogeneously applied although, a step at the cap-body junction was observed regardless the banding (Figure 2). The banded and not-banded capsules showed gastro-resistance even at low level of coating. However, following storage, the enteric functionality was fully maintained in the not-banded capsules filled at high level whereas, the capsules filled at low level failed the test in acid conditions (Figure 3). In general, the release of niacin was completed in phosphate buffer pH 6.8 within 60 minutes and, the dissolution rate increased as the level of coating and the capsule weight was low. Within 15 minutes testing in phosphate buffer, only a limited number of capsules started to dissolve. These capsules were not-banded, low-filled and low-coated and, following two months storage, a higher number of capsules, including high-filled capsules, started to release within this timeframe.
Conclusion: Gastro-resistance and full API release in phosphate buffer pH 6.8 was achieved with both banded and not-banded capsules. In general, the enteric coat applied to the capsules guaranteed the closure of the gap at the cap-body junction. Thus, the capsule banding step can be by-passed if careful assessment of the level of coat is conducted as well as appropriate testing of the dosage form throughout storage. The coating process parameters were selected to provide suitable fluidization of the capsules in the rotating pan and obtain a visually homogeneous coat. Faster dissolution rate and reduced gastro-resistance was observed in low-filled capsules in comparison to high-filled capsules. This trend was linked to the application of the enteric film during coating and the fluidization of the capsules in the pan. Therefore, the coating process parameters have to be selected also on the basis of both the individual weight of the capsule and the batch size to be coated. This is particularly relevant if not-banded capsules are to be coated.
References: 1. Fu M, Blechar JA, Sauer A, Al-Gousous J, Langguth P. In Vitro Evaluation of Enteric-Coated HPMC Capsules-Effect of Formulation Factors on Product Performance. Pharmaceutics. 2020 Jul 23;12(8):696.
2. Evonik EUDRAGIT® Application Guidelines; Evonik Nutrition & Care GmbH: Darmstadt, Germany, 2022; Available online: https://oncare.evonik.com/ (accessed on 07 July 2022).
.jpg)
Figure 1: manufacturing process details

Figure 2: Coated capsules

Figure 3: Dissolution testing
