Back
Purpose: Nano-emulsions (NEs) are oil-in-water (O/W) or water-in-oil (W/O) dispersion of two immiscible liquids stabilized using a surfactant. Their mean droplet size of < 500 nm renders them with a clear or hazy appearance in contrast to the milky white colour of coarse emulsion. Moreover, the small droplet size of NEs discourages conventional destabilization processes like creaming, sedimentation, and coalescence to yield high solubilization capacity and kinetic stability1. NEs’ applications as drug delivery systems are limited due to high concentration of surfactant. In this study, we aim to formulate a stable nano-emulsion with acceptable levels of surfactant to encapsulate celastrol (Cela), an herbal drug. Celastrol, a principal bioactive ingredient of Tripterygium wilfordii, also known as Thunder God Vine or Seven-step Vine has been found to have multiple promising biological and pharmacological activities2. It has been shown to have anti-cancer, anti-inflammatory, anti-diabetic, anti-microbial efficacy, along with utility in treating cardiovascular and even CNS disorders. Cela induces apoptosis and autophagy via the ROS/JNK signalling pathway and inhibits dopaminergic neuronal death of Parkinson's disease through activating mitophagy. However, its strong hydrophobicity i.e., low aqueous solubility, poor corresponding bioavailability, short biological half-life and narrow therapeutic window have deterred its clinical translation. Therefore, to overcome these shortcomings of Cela, we aim to encapsulate it efficiently in a stable nano-emulsion which can be used for treatment above mentioned pharmacological conditions.
Methods: A clear stable Cela-NE was prepared using Capmul MCM, Tween 80, Transcutol HP and deionized water as oil, surfactant, co-surfactant and aqueous phase, respectively. Briefly Cela was solubilized overnight in Capmul MCM (25%) followed by gentle vortexing with Smix (25%) (Tween 80: Transcutol HP 4:1) to form the oil phase. The volume of aqueous phase (50%) was determined using water titration method, where small volume of water was added dropwise to the oil phase, until it turned hazy. The resulting Cela-NE was evaluated for % drug encapsulation, globule size estimation and zeta potential. Preliminary in-vitro studies in lung cancer cells (A549) were performed to test the cytotoxic potential of Cela-NE against its plain drug counterpart using MTT assay.
Results: The physical appearance of both blank and Cela-loaded NE was clear, although Cela-loaded NE was orange in color attributed by the inherent colour of Cela. % Encapsulation of Cela was evaluated to be 97.2±1.8 % which can be explained by the high solubility of lipophilic Cela in oil phase. Additionally, the presence of surfactant-co-surfactant blend may also aid in solubilizing the drug. The mean globule size was found be 201.4±3.7 nm with a PDI of 0.4±0.1, while the zeta potential was -15.7±0.2 mV indicating stability (Table 1). The in-vitro studies performed in A549 non-small cell lung cancer yielded positive results where the Cela-NE showed better cytotoxicity (IC50 0.3±0.1 µM) as compared with its plain drug counterpart (IC50 1.2 ± 0.2 µM) (Fig. 1).
Conclusion: These preliminary results corroborate NE to be a promising delivery system, that can be employed for repurposing of Celastrol for potential pharmacological actions. Future experiments involving comprehensive study of drug release mechanism, in-vitro mechanism of uptake etc., and also in-vivo response would further help in understanding its potential as an effective drug delivery system.
References: 1. Singh, Yuvraj; Meher, Jaya Gopal; Raval, Kavit; Khan, Farooq Ali; Chaurasia, Mohini; Jain, Nitin K.; Chourasia, Manish K. (2017). Nanoemulsion: Concepts, development and applications in drug delivery. Journal of Controlled Release, 252(), 28–49. doi:10.1016/j.jconrel.2017.03.008
2. Hou, Wei; Liu, Bo; Xu, Hongtao (2020). Celastrol: Progresses in structure-modifications, structure-activity relationships, pharmacology and toxicology. European Journal of Medicinal Chemistry, 189(), 112081–. doi:10.1016/j.ejmech.2020.112081

Table 1: % Encapsulation of Celastrol in the NE and its physical characterization
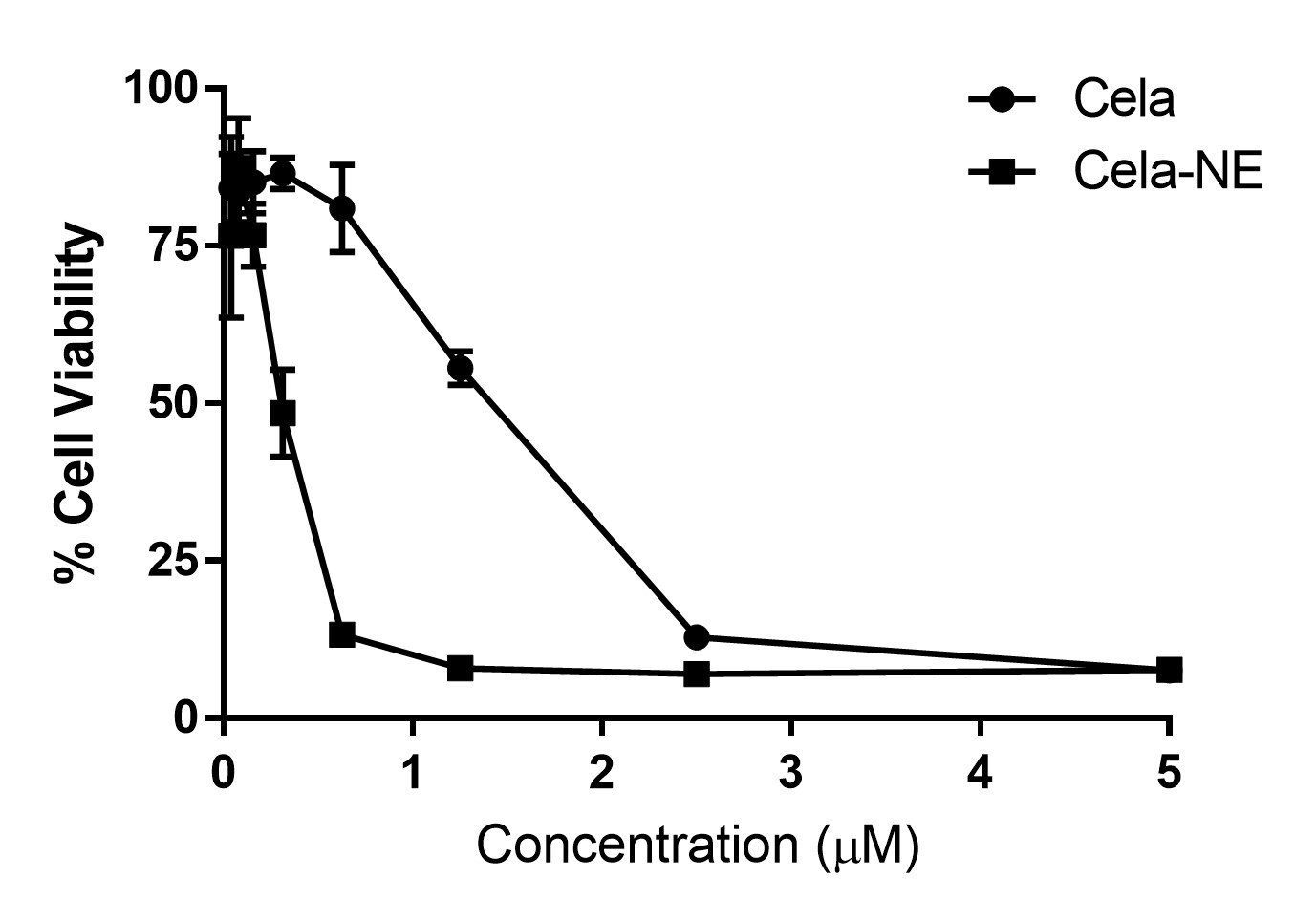
Fig. 1. Cytotoxicity assay of plain drug Cela and Cela-NE at same concentrations
Formulation and Delivery - Chemical - Drug Delivery, Devices, and Drug Device
Category: Poster Abstract
(T1430-04-22) Developing an Inhaled Nano-Emulsion for Repurposing an Herbal Drug for Non-small Cell Lung Cancer
Tuesday, October 18, 2022
2:30 PM – 3:30 PM ET
- MQ
Mural Quadros, MS
St. John's University
Queens, New York, United States - MQ
Mural Quadros, MS
St. John's University
Queens, New York, United States
Presenting Author(s)
Main Author(s)
Purpose: Nano-emulsions (NEs) are oil-in-water (O/W) or water-in-oil (W/O) dispersion of two immiscible liquids stabilized using a surfactant. Their mean droplet size of < 500 nm renders them with a clear or hazy appearance in contrast to the milky white colour of coarse emulsion. Moreover, the small droplet size of NEs discourages conventional destabilization processes like creaming, sedimentation, and coalescence to yield high solubilization capacity and kinetic stability1. NEs’ applications as drug delivery systems are limited due to high concentration of surfactant. In this study, we aim to formulate a stable nano-emulsion with acceptable levels of surfactant to encapsulate celastrol (Cela), an herbal drug. Celastrol, a principal bioactive ingredient of Tripterygium wilfordii, also known as Thunder God Vine or Seven-step Vine has been found to have multiple promising biological and pharmacological activities2. It has been shown to have anti-cancer, anti-inflammatory, anti-diabetic, anti-microbial efficacy, along with utility in treating cardiovascular and even CNS disorders. Cela induces apoptosis and autophagy via the ROS/JNK signalling pathway and inhibits dopaminergic neuronal death of Parkinson's disease through activating mitophagy. However, its strong hydrophobicity i.e., low aqueous solubility, poor corresponding bioavailability, short biological half-life and narrow therapeutic window have deterred its clinical translation. Therefore, to overcome these shortcomings of Cela, we aim to encapsulate it efficiently in a stable nano-emulsion which can be used for treatment above mentioned pharmacological conditions.
Methods: A clear stable Cela-NE was prepared using Capmul MCM, Tween 80, Transcutol HP and deionized water as oil, surfactant, co-surfactant and aqueous phase, respectively. Briefly Cela was solubilized overnight in Capmul MCM (25%) followed by gentle vortexing with Smix (25%) (Tween 80: Transcutol HP 4:1) to form the oil phase. The volume of aqueous phase (50%) was determined using water titration method, where small volume of water was added dropwise to the oil phase, until it turned hazy. The resulting Cela-NE was evaluated for % drug encapsulation, globule size estimation and zeta potential. Preliminary in-vitro studies in lung cancer cells (A549) were performed to test the cytotoxic potential of Cela-NE against its plain drug counterpart using MTT assay.
Results: The physical appearance of both blank and Cela-loaded NE was clear, although Cela-loaded NE was orange in color attributed by the inherent colour of Cela. % Encapsulation of Cela was evaluated to be 97.2±1.8 % which can be explained by the high solubility of lipophilic Cela in oil phase. Additionally, the presence of surfactant-co-surfactant blend may also aid in solubilizing the drug. The mean globule size was found be 201.4±3.7 nm with a PDI of 0.4±0.1, while the zeta potential was -15.7±0.2 mV indicating stability (Table 1). The in-vitro studies performed in A549 non-small cell lung cancer yielded positive results where the Cela-NE showed better cytotoxicity (IC50 0.3±0.1 µM) as compared with its plain drug counterpart (IC50 1.2 ± 0.2 µM) (Fig. 1).
Conclusion: These preliminary results corroborate NE to be a promising delivery system, that can be employed for repurposing of Celastrol for potential pharmacological actions. Future experiments involving comprehensive study of drug release mechanism, in-vitro mechanism of uptake etc., and also in-vivo response would further help in understanding its potential as an effective drug delivery system.
References: 1. Singh, Yuvraj; Meher, Jaya Gopal; Raval, Kavit; Khan, Farooq Ali; Chaurasia, Mohini; Jain, Nitin K.; Chourasia, Manish K. (2017). Nanoemulsion: Concepts, development and applications in drug delivery. Journal of Controlled Release, 252(), 28–49. doi:10.1016/j.jconrel.2017.03.008
2. Hou, Wei; Liu, Bo; Xu, Hongtao (2020). Celastrol: Progresses in structure-modifications, structure-activity relationships, pharmacology and toxicology. European Journal of Medicinal Chemistry, 189(), 112081–. doi:10.1016/j.ejmech.2020.112081

Table 1: % Encapsulation of Celastrol in the NE and its physical characterization

Fig. 1. Cytotoxicity assay of plain drug Cela and Cela-NE at same concentrations
