Back
Purpose: Cannabidiol, a non-psychoactive phytoconstituent, from Cannabis sativa, has reported to possess neuroprotective, anti-inflammatory and anti-oxidant potential. Studies have shown its ability to reduce amyloid loads as well as promote hippocampal neurogenesis. Cannabidiol, thus, appears as a promising therapeutic option against AD. ApoE is an apolipoprotein having roles in circulation and metabolism of lipids. ApoE exists in three most common isoforms i.e. ApoE2, ApoE3 and ApoE4 differing from each other at amino acid residues 112 and 158. The gene encoding ApoE has been shown as one of the strongest risk factor for development of AD. Subjects with ApoE4 allele are more likely to be predisposed with AD in their life whereas the ones with ApoE2 allele are at lower risk of developing AD and it also delays the onset of the disease. Patients with ApoE2 allele have lower level of amyloid plaques and few neurofibrillary tangles in their brain. This suggests ApoE2 expression in brain could be of therapeutic value to AD patients. As a result, we hypothesize that delivery of Cannabidiol and plasmid ApoE2 reduces the pathological features in AD patients and improve their cognitive ability. This study, thus, aims at developing formulation that can deliver both cannabidiol and plasmid ApoE2 to the brain.
Methods: Thin film lipid hydration method was employed to formulate liposomes having lipids DOPE: DOTAP: DSPE PEG: Cholesterol in molar ratio of 45:45:8:2. The size, PDI and zeta potential were measured using zetasizer. Cannabidiol was dissolved in lipid mixture during film formation at 10 % w/w of lipid weight whereas plasmid ApoeE2 complexed with chitosan (N:P ratio of 5) was added during the hydration. The unencapsulated drug was separated after passing through sephadex G-100 gel column. Reverse phase chromatography was performed to analyze Cannabidiol. C18 column was employed having mobile phase composition of acetonitrile: methanol: water at ratio of 7:1:2 with flow rate of 0.8 ml/minute. The eluted drug was analyzed at wavelength of 220 nm. MTT assay was performed to determine the cell viability of in response to cannabidiol exposure. Two cell lines b.END3 and Immortalized Microglial (IMG) were selected for the experiment. 5000 cells per well were plated overnight in 96 well plates and treated with different concentration of cannabidiol the following day. After 24 hours of treatment, the media was removed and 10ul of 5 mg/ml MTT was added to each well. The formazan crystal formed after 3 hours of treatment were dissolved in DMSO and relative viability was calculated. 1.5 ml Cannabidiol encapsulated liposomes were placed in a dialysis membrane (MWCO 12 – 14 kD) and sealed tightly. The dialysis tube was then immersed in 50 ml of phosphate buffer (pH 7.4) with 2% Tween-80 and incubated at 37±0.5°C with oscillation of 50 rpm. 1 ml of sample was withdrawn at selected time points and replaced with fresh buffer solution. The aliquots were analyzed using HPLC for drug release.
Results: Cananbidiol liposomes were prepared of size 179.7 ± 15.06 nm with PDI 0.226 ± 0.020 and zeta potential 10.1 ± 1.18 mV. The formulation was able to encapsulate 70.64 ± 3.56 % of plasmid DNA. HPLC peak was obtained with the applied method at retention time of 8.42 min and concentration as low as 100 ng/ml was estimated with this method (Figure 1). Encapsulation efficiency of the Cannabidiol was obtained as 79.28 ± 10.21 %. MTT assay performed on b.END3 and IMG cells demonstrated concentration above 10μM and 7.5 μM respectively maintained cell viability above 80% (Figure 2). The formulation successfully released 75.05 ± 6.22 % of drug in 2 days time period (Figure 3).
Conclusion: Liposome formulation was successfully formulated to encapsulate the drug Cannabidiol as well as pApoE2. HPLC method was developed to quantify the Cannabidiol in related experiments. The formulation was able to release approximately three quarter of a drug in 48 hours. MTT assay revealed working concentrations of 10μM and 7.5 μM , respectively, for the b.END3 and IMG cell line for future in vitro experiments. The efficacy of these formulation will be tested against Alzheimers disease through appropriate in vitro and in vivo experiments
Acknowledgements: This research was supported by the National Institute of Health (NIH) grant RF1 AG068034
.jpg)
Figure 1: HPLC chromatogram of Cannabidiol obtained using reverse phase chromatography with mobile phase composition of acetonitrile: methanol: water at 7:1:2 ratio, flow rate 0.8 ml/min and UV detector wavelength of 220 nm
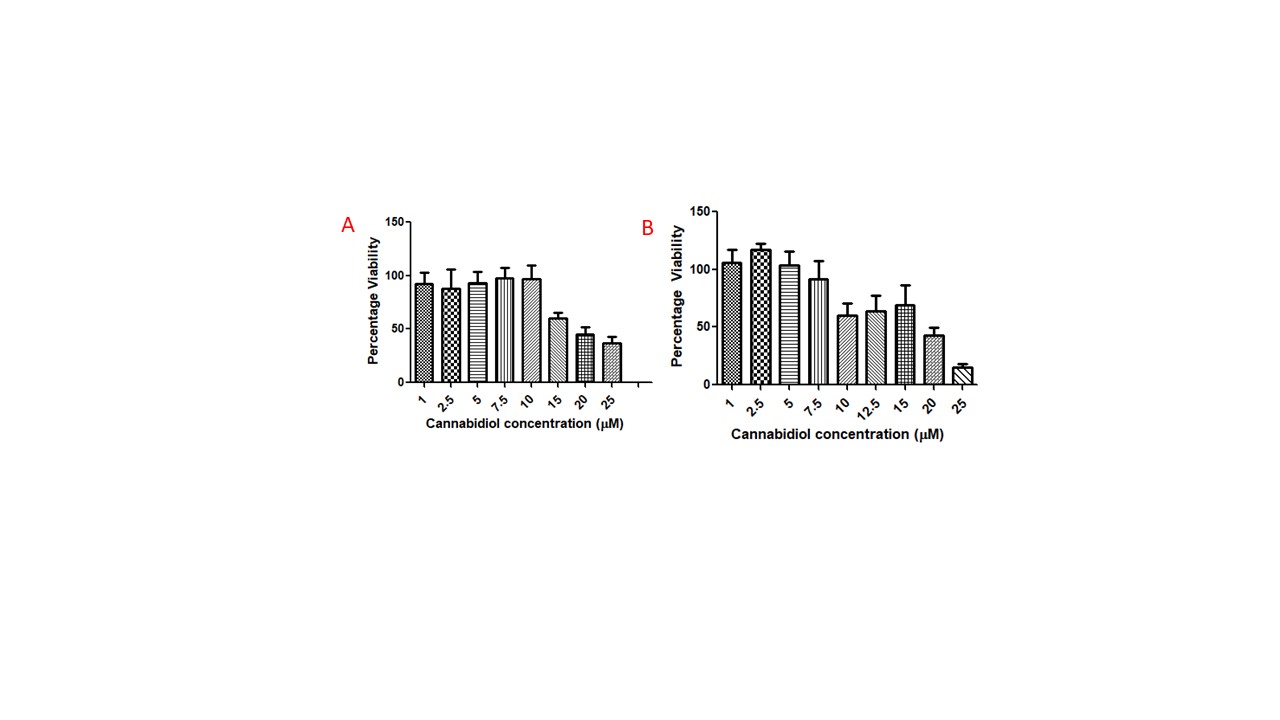
Figure 2: MTT assay of Cannabidiol on b.END3 cells (A) and IMG cell line (B) 24 hours after the treatment. The data represents mean mean ± SD (n = 4)
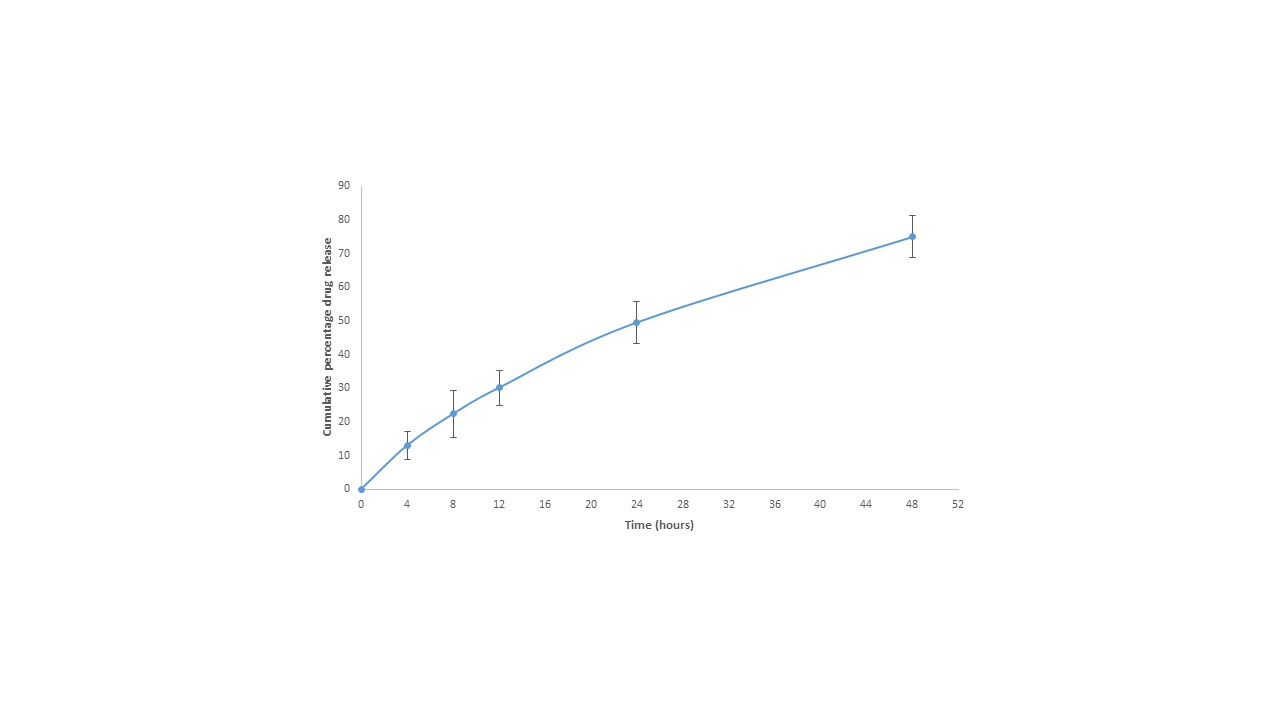
Figure 3: Cumulative percentage release of Cannabidiol in release medium of 2% Tween-80 at release medium of pH 7.4. The data represents mean mean ± SD (n = 3)
Formulation and Delivery - Chemical - Drug Delivery
Category: Poster Abstract
(M0930-12-67) Liposome as a Carrier for Delivery of Cannabidiol and pAPOE2 for the Management of Alzheimer's Disease
Monday, October 17, 2022
9:30 AM – 10:30 AM ET
- BC
Bivek Chaulagain, MS
North Dakota State University
Fargo, North Dakota, United States - JS
Jagdish Singh, Ph.D.
North Dakota State University
Fargo, North Dakota, United States
Presenting Author(s)
Main Author(s)
Purpose: Cannabidiol, a non-psychoactive phytoconstituent, from Cannabis sativa, has reported to possess neuroprotective, anti-inflammatory and anti-oxidant potential. Studies have shown its ability to reduce amyloid loads as well as promote hippocampal neurogenesis. Cannabidiol, thus, appears as a promising therapeutic option against AD. ApoE is an apolipoprotein having roles in circulation and metabolism of lipids. ApoE exists in three most common isoforms i.e. ApoE2, ApoE3 and ApoE4 differing from each other at amino acid residues 112 and 158. The gene encoding ApoE has been shown as one of the strongest risk factor for development of AD. Subjects with ApoE4 allele are more likely to be predisposed with AD in their life whereas the ones with ApoE2 allele are at lower risk of developing AD and it also delays the onset of the disease. Patients with ApoE2 allele have lower level of amyloid plaques and few neurofibrillary tangles in their brain. This suggests ApoE2 expression in brain could be of therapeutic value to AD patients. As a result, we hypothesize that delivery of Cannabidiol and plasmid ApoE2 reduces the pathological features in AD patients and improve their cognitive ability. This study, thus, aims at developing formulation that can deliver both cannabidiol and plasmid ApoE2 to the brain.
Methods: Thin film lipid hydration method was employed to formulate liposomes having lipids DOPE: DOTAP: DSPE PEG: Cholesterol in molar ratio of 45:45:8:2. The size, PDI and zeta potential were measured using zetasizer. Cannabidiol was dissolved in lipid mixture during film formation at 10 % w/w of lipid weight whereas plasmid ApoeE2 complexed with chitosan (N:P ratio of 5) was added during the hydration. The unencapsulated drug was separated after passing through sephadex G-100 gel column. Reverse phase chromatography was performed to analyze Cannabidiol. C18 column was employed having mobile phase composition of acetonitrile: methanol: water at ratio of 7:1:2 with flow rate of 0.8 ml/minute. The eluted drug was analyzed at wavelength of 220 nm. MTT assay was performed to determine the cell viability of in response to cannabidiol exposure. Two cell lines b.END3 and Immortalized Microglial (IMG) were selected for the experiment. 5000 cells per well were plated overnight in 96 well plates and treated with different concentration of cannabidiol the following day. After 24 hours of treatment, the media was removed and 10ul of 5 mg/ml MTT was added to each well. The formazan crystal formed after 3 hours of treatment were dissolved in DMSO and relative viability was calculated. 1.5 ml Cannabidiol encapsulated liposomes were placed in a dialysis membrane (MWCO 12 – 14 kD) and sealed tightly. The dialysis tube was then immersed in 50 ml of phosphate buffer (pH 7.4) with 2% Tween-80 and incubated at 37±0.5°C with oscillation of 50 rpm. 1 ml of sample was withdrawn at selected time points and replaced with fresh buffer solution. The aliquots were analyzed using HPLC for drug release.
Results: Cananbidiol liposomes were prepared of size 179.7 ± 15.06 nm with PDI 0.226 ± 0.020 and zeta potential 10.1 ± 1.18 mV. The formulation was able to encapsulate 70.64 ± 3.56 % of plasmid DNA. HPLC peak was obtained with the applied method at retention time of 8.42 min and concentration as low as 100 ng/ml was estimated with this method (Figure 1). Encapsulation efficiency of the Cannabidiol was obtained as 79.28 ± 10.21 %. MTT assay performed on b.END3 and IMG cells demonstrated concentration above 10μM and 7.5 μM respectively maintained cell viability above 80% (Figure 2). The formulation successfully released 75.05 ± 6.22 % of drug in 2 days time period (Figure 3).
Conclusion: Liposome formulation was successfully formulated to encapsulate the drug Cannabidiol as well as pApoE2. HPLC method was developed to quantify the Cannabidiol in related experiments. The formulation was able to release approximately three quarter of a drug in 48 hours. MTT assay revealed working concentrations of 10μM and 7.5 μM , respectively, for the b.END3 and IMG cell line for future in vitro experiments. The efficacy of these formulation will be tested against Alzheimers disease through appropriate in vitro and in vivo experiments
Acknowledgements: This research was supported by the National Institute of Health (NIH) grant RF1 AG068034
.jpg)
Figure 1: HPLC chromatogram of Cannabidiol obtained using reverse phase chromatography with mobile phase composition of acetonitrile: methanol: water at 7:1:2 ratio, flow rate 0.8 ml/min and UV detector wavelength of 220 nm

Figure 2: MTT assay of Cannabidiol on b.END3 cells (A) and IMG cell line (B) 24 hours after the treatment. The data represents mean mean ± SD (n = 4)

Figure 3: Cumulative percentage release of Cannabidiol in release medium of 2% Tween-80 at release medium of pH 7.4. The data represents mean mean ± SD (n = 3)
