Back
Purpose: The purpose of this study was to develop a fixed dose combination (FDC) with sustained released acetaminophen (AAP) and solubilized ibuprofen (IBF) in a dosage form by double-melt extrusion (DME) technology.
Methods: Hot-melt extrusion (HME) was performed using co-rotating twin-screw extruders (11 mm Process 11, ThermoFisher Scientific, Pittsburgh, PA, USA). The first melt extrudate was prepared with AAP and a polymer with a relatively higher Tg such as polyvinylpyrrolidone K-30 (PVP K-30) (AAP:PVP K-30=1:4). First melt extrudate was obtained using a screw speed of 50 rpm and a process temperature of 170 ℃. The second melt extrudate was obtained using the milled first extrudate, IBF, and polymers with a lower Tg, such as polyvinylpyrrolidone VA64 (PVP VA64) and polyethylene glycol (PEG) 6000 (first extrudate:IBF:PVP VA64:PEG 6000=5:1:3.5:0.5). The second melt extrudate was obtained using a screw speed of 50 rpm and a process temperature of 80 ℃. After obtaining the melt extrudate, milling and sieving processes were applied to obtain granule sizes of 250–600 μm. The granules were then compressed using a single tableting machine. Thermogravimetric analysis (TGA), Differential scanning calorimetry (DSC), Powder X-ray diffraction (PXRD) were performed to analyze the physicochemical properties of the hot melt extrudates. A dissolution test was performed in artificial gastric fluid (pH 1.2, 900 mL) at 37±0.5 ℃ using the paddle method (USP apparatus Ⅱ), with a paddle rotating at 50 rpm. To understand the mechanism of DME, a very low amount of coumarin-6 (for AAP) or rhodamine-B (for IBF) fluorescent dye was added to visualize distribution images using a confocal laser scanning microscope (CLSM).
Results: The dissolution tests showed that pure IBF had the lowest dissolution rate of 10.6% in 120 minutes. DME, single-melt extrusion (SME), and the physical mixture (PM) showed significantly improved IBF release behaviors, with 4.0-fold, 4.5-fold and 3.7-fold increases compared to that of pure IBF. DME and SME had a higher dissolution rate compared to that of the PM, reaching 42.47% and 47.13% in 120 min (Fig 1.a). Pure AAP showed the highest dissolution rate of 98.72% in 120 minutes. DME showed a more retarded AAP release compared to that of the SME and PM, approaching 65.90% in 120 min. (Fig 1.b). Physicochemical properties were confirmed by TGA (Fig 2.a), DSC (Fig 2.b), and PXRD (Fig 2.c). Pure AAP, IBF, and PM showed crystalline structure, but all extrudates were changed to amorphous states. In the PM, AAP-coumarin-6 and IBF-rhodamine-B were irregularly dispersed through the CLSM images, and yellowish portions were revealed by overlapping fluorescent dyes (Fig 3.a-d). DME could be confirmed that the first extrudate was coated with the second extrudate through the CLSM image (Fig 3.e-h).
Conclusion: The novelty of the current investigation was to develop DME in two stages via a double extrusion process. It was confirmed that the crystalline AAP and IBF were changed to amorphous states by HME. Using the DME process, it was possible to prepare a dual drug release system with AAP showing sustained release and IBF showing solubilized and immediate release behavior in one dosage form simultaneously.
Acknowledgments: This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (NRF-2022R1F1A1063127).
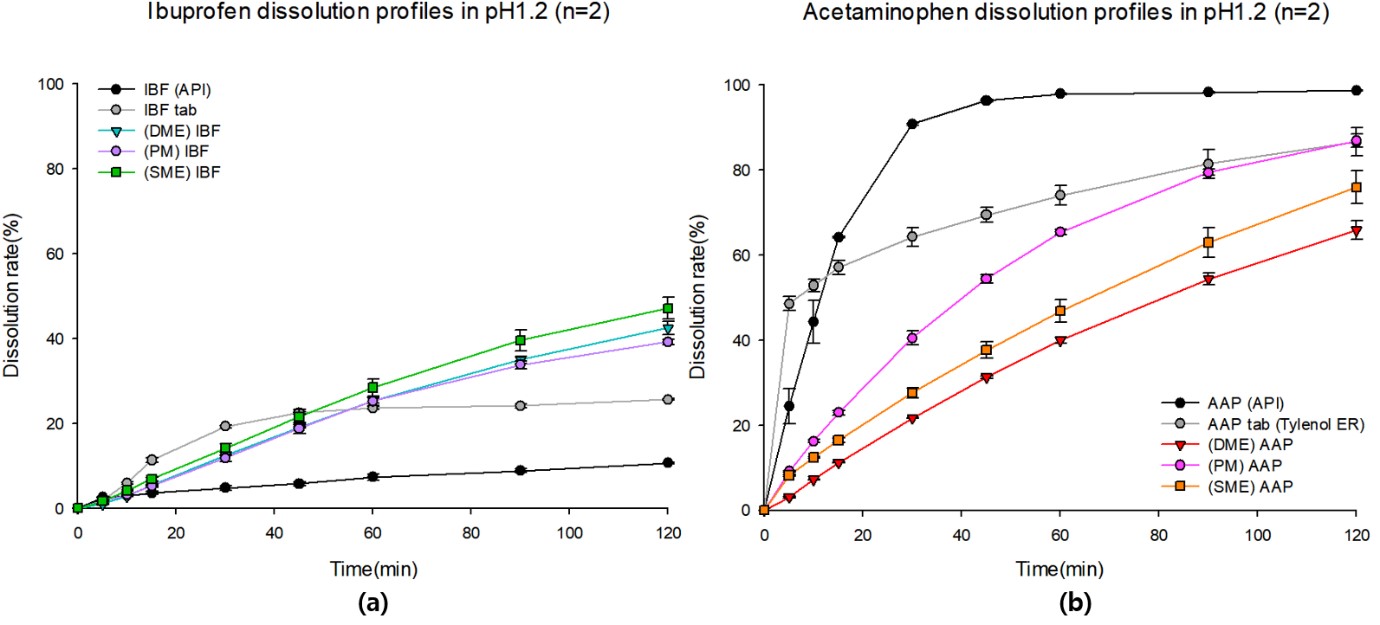
Figure 1. Dissolution profiles in pH 1.2 solution; (a) IBF and (b) AAP. IBF, ibuprofen; AAP, acetaminophen.
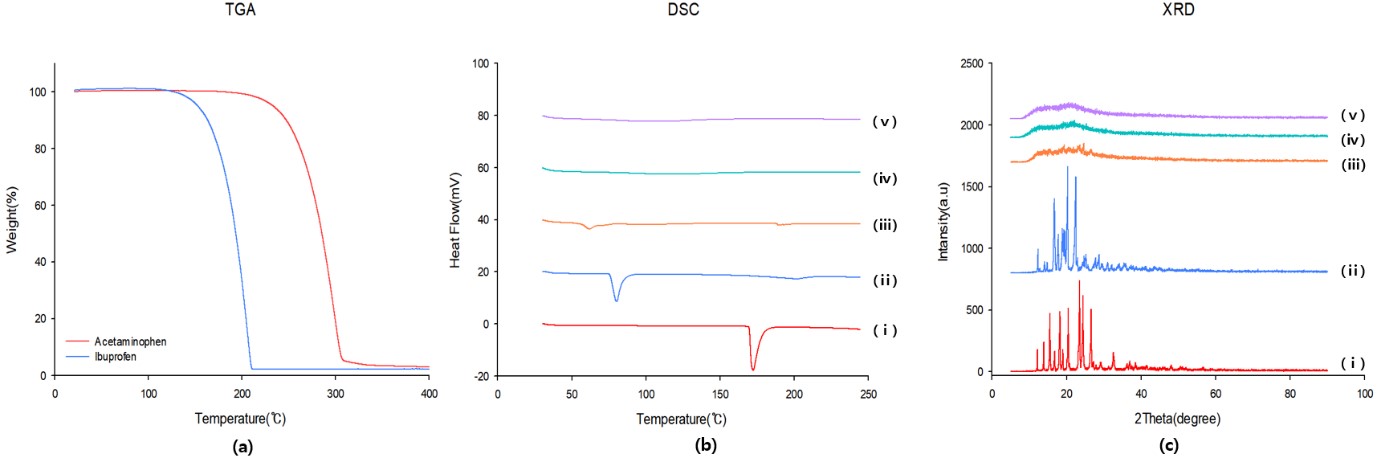
Figure 2. (a) TGA thermograms of the samples, (b) DSC spectra of the samples, (b) XRD diagrams of the samples; pure material (ⅰ) AAP, (ⅱ) IBF, (ⅲ) PM, (ⅳ) SME, and (ⅴ) DME. AAP, acetaminophen; IBF, ibuprofen; PM, physical mixture; SME, single-melt extrusion; DME, double-melt extrusion.
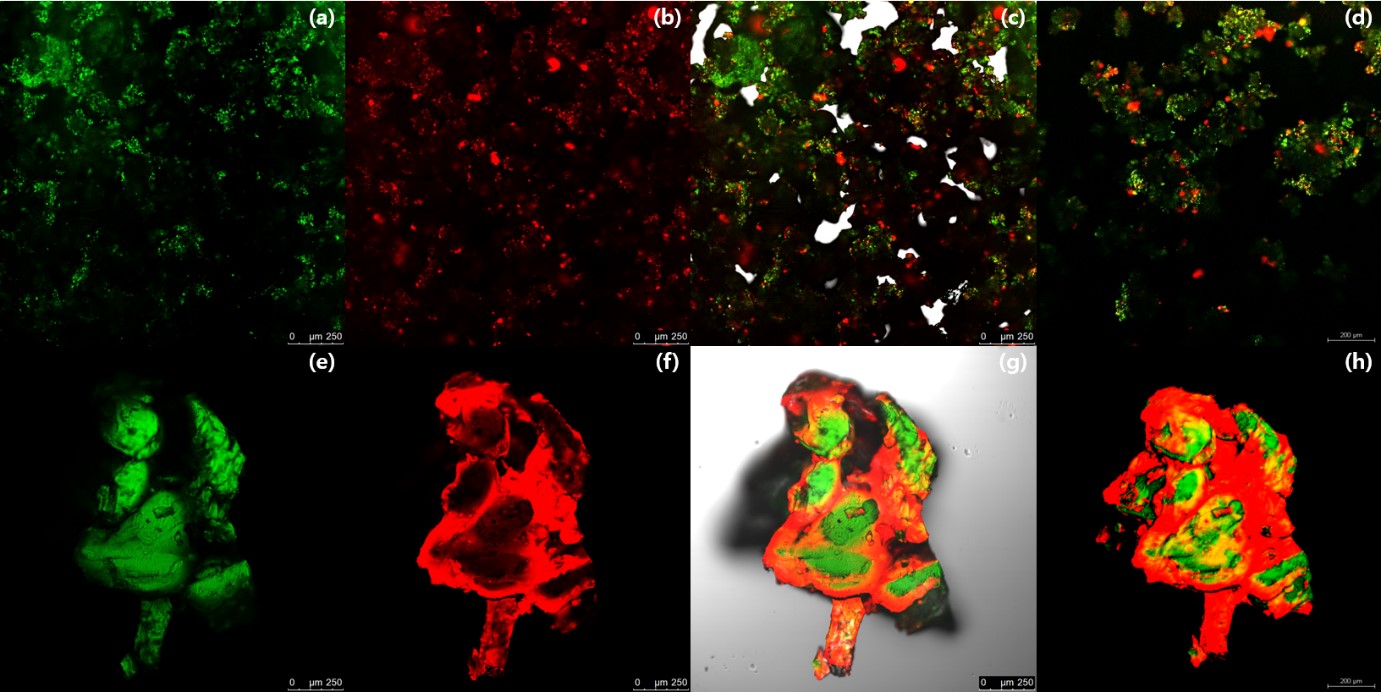
Figure 3. Confocal laser scanning microscope images of physical mixture (a) AAP-coumarin-6 slice view, (b) IBF-rhodamine-B slice view, (c) AAP-coumarine-6+IBF-rhodamine-B slice view, and (d) AAP-coumarine-6+IBF-rhodamine-B sliced 3D view; double melt extrusion, (e) AAP-coumarin-6 slice view, (f) IBF-rhodamine-B slice view, (g) AAP-coumarine-6+IBF-rhodamine-B slice view, and (h) AAP-coumarine-6+IBF-rhodamine-B sliced 3D view. AAP, acetaminophen; IBF, ibuprofen.
Formulation and Delivery - Chemical - Formulation
Category: Late Breaking Poster Abstract
(T1530-12-71) Development of a Fixed Dose Combinations Using Double Melt Extrusion Technology
Tuesday, October 18, 2022
3:30 PM – 4:30 PM ET
- HS
Hee-Kyung Seo
Sahmyook University
Seoul, Seoul-t'ukpyolsi, Republic of Korea - JP
Jun-Bom Park, Ph.D.
Sahmyook University
Seoul, Seoul-t'ukpyolsi, Republic of Korea
Presenting Author(s)
Main Author(s)
Purpose: The purpose of this study was to develop a fixed dose combination (FDC) with sustained released acetaminophen (AAP) and solubilized ibuprofen (IBF) in a dosage form by double-melt extrusion (DME) technology.
Methods: Hot-melt extrusion (HME) was performed using co-rotating twin-screw extruders (11 mm Process 11, ThermoFisher Scientific, Pittsburgh, PA, USA). The first melt extrudate was prepared with AAP and a polymer with a relatively higher Tg such as polyvinylpyrrolidone K-30 (PVP K-30) (AAP:PVP K-30=1:4). First melt extrudate was obtained using a screw speed of 50 rpm and a process temperature of 170 ℃. The second melt extrudate was obtained using the milled first extrudate, IBF, and polymers with a lower Tg, such as polyvinylpyrrolidone VA64 (PVP VA64) and polyethylene glycol (PEG) 6000 (first extrudate:IBF:PVP VA64:PEG 6000=5:1:3.5:0.5). The second melt extrudate was obtained using a screw speed of 50 rpm and a process temperature of 80 ℃. After obtaining the melt extrudate, milling and sieving processes were applied to obtain granule sizes of 250–600 μm. The granules were then compressed using a single tableting machine. Thermogravimetric analysis (TGA), Differential scanning calorimetry (DSC), Powder X-ray diffraction (PXRD) were performed to analyze the physicochemical properties of the hot melt extrudates. A dissolution test was performed in artificial gastric fluid (pH 1.2, 900 mL) at 37±0.5 ℃ using the paddle method (USP apparatus Ⅱ), with a paddle rotating at 50 rpm. To understand the mechanism of DME, a very low amount of coumarin-6 (for AAP) or rhodamine-B (for IBF) fluorescent dye was added to visualize distribution images using a confocal laser scanning microscope (CLSM).
Results: The dissolution tests showed that pure IBF had the lowest dissolution rate of 10.6% in 120 minutes. DME, single-melt extrusion (SME), and the physical mixture (PM) showed significantly improved IBF release behaviors, with 4.0-fold, 4.5-fold and 3.7-fold increases compared to that of pure IBF. DME and SME had a higher dissolution rate compared to that of the PM, reaching 42.47% and 47.13% in 120 min (Fig 1.a). Pure AAP showed the highest dissolution rate of 98.72% in 120 minutes. DME showed a more retarded AAP release compared to that of the SME and PM, approaching 65.90% in 120 min. (Fig 1.b). Physicochemical properties were confirmed by TGA (Fig 2.a), DSC (Fig 2.b), and PXRD (Fig 2.c). Pure AAP, IBF, and PM showed crystalline structure, but all extrudates were changed to amorphous states. In the PM, AAP-coumarin-6 and IBF-rhodamine-B were irregularly dispersed through the CLSM images, and yellowish portions were revealed by overlapping fluorescent dyes (Fig 3.a-d). DME could be confirmed that the first extrudate was coated with the second extrudate through the CLSM image (Fig 3.e-h).
Conclusion: The novelty of the current investigation was to develop DME in two stages via a double extrusion process. It was confirmed that the crystalline AAP and IBF were changed to amorphous states by HME. Using the DME process, it was possible to prepare a dual drug release system with AAP showing sustained release and IBF showing solubilized and immediate release behavior in one dosage form simultaneously.
Acknowledgments: This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (NRF-2022R1F1A1063127).

Figure 1. Dissolution profiles in pH 1.2 solution; (a) IBF and (b) AAP. IBF, ibuprofen; AAP, acetaminophen.

Figure 2. (a) TGA thermograms of the samples, (b) DSC spectra of the samples, (b) XRD diagrams of the samples; pure material (ⅰ) AAP, (ⅱ) IBF, (ⅲ) PM, (ⅳ) SME, and (ⅴ) DME. AAP, acetaminophen; IBF, ibuprofen; PM, physical mixture; SME, single-melt extrusion; DME, double-melt extrusion.

Figure 3. Confocal laser scanning microscope images of physical mixture (a) AAP-coumarin-6 slice view, (b) IBF-rhodamine-B slice view, (c) AAP-coumarine-6+IBF-rhodamine-B slice view, and (d) AAP-coumarine-6+IBF-rhodamine-B sliced 3D view; double melt extrusion, (e) AAP-coumarin-6 slice view, (f) IBF-rhodamine-B slice view, (g) AAP-coumarine-6+IBF-rhodamine-B slice view, and (h) AAP-coumarine-6+IBF-rhodamine-B sliced 3D view. AAP, acetaminophen; IBF, ibuprofen.
