Back
Purpose: Robust SEDDS formulation development is based on in-depth analysis and understanding of a given API’s physicochemical and biological characteristics and, if appropriate, the consequent selection of specific lipid-based excipients known to have physiological effects1.The purpose of this study was to design and develop lipid formulations which address the poor aqueous solubility and low bioavailability, for a BCS class II new chemical entity (NCE) with intermediate lipophilicity (logP 4) and low aqueous solubility ( < 2 µg/ml). A solution and suspension approach were investigated to assess the impact of different formulation dynamics and the effect of increasing drug loading in-vivo.
Methods: SEDDS were designed using API solubility and chemical stability data in a range of oils, co-surfactants and surfactants, at a range of ratios. Solution formulations were initially selected based on their ability to solubilise the API at the target dose, and physical formulation stability and suspension formulations were selected based on the physical nature of the formulations. Dispersion behaviour was assessed in water, focussing on emulsion stability and risk of compound precipitation over six hours. Based on the dispersion performance, concept formulations were progressed onto in vitro lipolysis assessment using the methods developed by the LFCS Consortium2 to understand the fate of the drug over time in simulated small intestinal conditions. Lead formulations were progressed to an in-vivo pharmacokinetic assessment in cynomolgus monkey, against the poorly digestible and surfactant based lead clinical formulation.
Results: Formulations were designed incorporating long chain lipids, low HLB surfactants and high HLB surfactants at a target drug loading of 2% w/w for solution formulations and 8% w/w for suspension formulations. Dispersion testing in water identified two lead solution formulations that formed stable emulsions, with no evidence of drug precipitation and two lead suspension formulations, based on the visual dispersion stability. One solution formulation and two suspension formulations were progressed to the lipolysis assessment, against the reference clinical formulation, prepared at 4% w/w. Lead formulations were composed of type III (20% lipid and 80% surfactants) and type IV lipid based formulations (100% water soluble surfactants). The solution and suspension formulations were readily digested within 60 minutes and measured solubilized drug concentrations in the aqueous-rich colloidal phase (Figure 1) were similar at 38 µg/ml (2% solution) and 43 µg/ml/34 µg/ml (8% suspensions). The reference clinical formulation at 4% API showed substantially lower aqueous concentrations at 11 µg/ml. The higher API concentrations in a readily available micellar form in the solution and suspension formulations suggested better absorption in vivo relative to the reference clinical formulation. The lead solution (2%), lead suspension (8%) and reference clinical formulation (4%) were then administered to cynomolgus monkeys and the results were dose normalized for comparison purposes. The solution formulation demonstrated a significant improvement in exposure with a Cmax of 1750 ng/ml, compared to the reference clinical formulation Cmax of 450 ng/ml and an increase in exposure was also shown with the suspension formulation (Cmax of 650 ng/ml).
Conclusion: Solution and suspension based SEDDS formulations consisting of long-chain lipids and cosurfactant/surfactant were developed to support API solubilization in the GI. Formulation performance was assessed in vitro, against the reference clinical formulation and the solution and suspension formulations demonstrated increased drug solubilisation in the aqueous phase during lipolysis. A significant increase in exposure was demonstrated in vivo using the solution based SEDDS formulation, demonstrating bioavailabiltiy enchancement using lipid based drug delivery.
References: 1. Stegemann, S et al. Self-Emulsifying Drug Delivery Systems: Fancing the bioavailability challenge in drug delviery. Pharmazeutische Industrie. 2009. 71(8). 1409-1416.
2. Williams, H et al. Toward the establishment of standardized in vitro tests for lipid-based formulations, part 1: Method parameterization and comparison of in vitro digestion profiles across a range of representative formulations. J. Pharm. Sci.
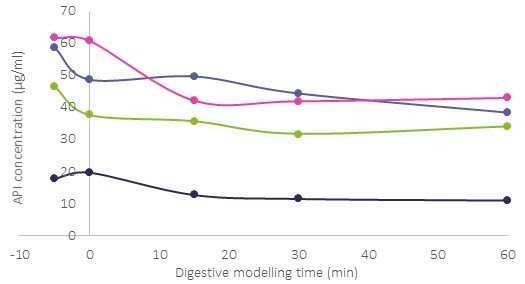
Figure 1 Solubilized compound concentration in the aqueous phase during in vitro digestion testing of a 2 % API solution formulation (purple) and 8 % API suspension formulations (pink and green) against the 4% clinical formulation (black).
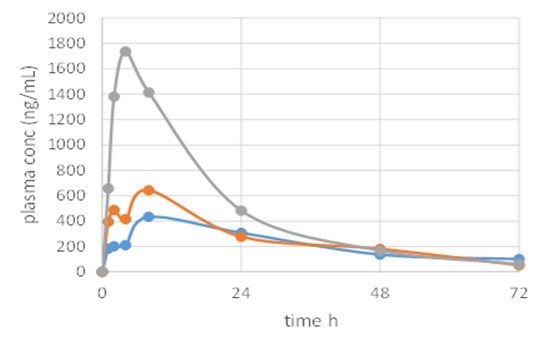
Figure 2: Plasma concentration following in vivo administration to cynomolgus monkeys of a 2 % API solution formulation (grey) and 8 % API suspension formulation (orange) against the 4% clinical formulation (blue), dose normailised.
Formulation and Delivery - Chemical - Formulation
Category: Late Breaking Poster Abstract
(M1230-04-23) Formulation Design, Characterisation and In Vivo Performance of Self-Emulsifying Drug Delivery Systems (SEDDS) for a BSC Class II NCE
Monday, October 17, 2022
12:30 PM – 1:30 PM ET
- DW
Dominic Walsh, BS
Formulation Group Leader
NextPharma
Livingston, Scotland, United Kingdom - JM
Jenifer Mains, Ph.D.
NextPharma
LIVINGSTON, Scotland, United Kingdom
Presenting Author(s)
Main Author(s)
Purpose: Robust SEDDS formulation development is based on in-depth analysis and understanding of a given API’s physicochemical and biological characteristics and, if appropriate, the consequent selection of specific lipid-based excipients known to have physiological effects1.The purpose of this study was to design and develop lipid formulations which address the poor aqueous solubility and low bioavailability, for a BCS class II new chemical entity (NCE) with intermediate lipophilicity (logP 4) and low aqueous solubility ( < 2 µg/ml). A solution and suspension approach were investigated to assess the impact of different formulation dynamics and the effect of increasing drug loading in-vivo.
Methods: SEDDS were designed using API solubility and chemical stability data in a range of oils, co-surfactants and surfactants, at a range of ratios. Solution formulations were initially selected based on their ability to solubilise the API at the target dose, and physical formulation stability and suspension formulations were selected based on the physical nature of the formulations. Dispersion behaviour was assessed in water, focussing on emulsion stability and risk of compound precipitation over six hours. Based on the dispersion performance, concept formulations were progressed onto in vitro lipolysis assessment using the methods developed by the LFCS Consortium2 to understand the fate of the drug over time in simulated small intestinal conditions. Lead formulations were progressed to an in-vivo pharmacokinetic assessment in cynomolgus monkey, against the poorly digestible and surfactant based lead clinical formulation.
Results: Formulations were designed incorporating long chain lipids, low HLB surfactants and high HLB surfactants at a target drug loading of 2% w/w for solution formulations and 8% w/w for suspension formulations. Dispersion testing in water identified two lead solution formulations that formed stable emulsions, with no evidence of drug precipitation and two lead suspension formulations, based on the visual dispersion stability. One solution formulation and two suspension formulations were progressed to the lipolysis assessment, against the reference clinical formulation, prepared at 4% w/w. Lead formulations were composed of type III (20% lipid and 80% surfactants) and type IV lipid based formulations (100% water soluble surfactants). The solution and suspension formulations were readily digested within 60 minutes and measured solubilized drug concentrations in the aqueous-rich colloidal phase (Figure 1) were similar at 38 µg/ml (2% solution) and 43 µg/ml/34 µg/ml (8% suspensions). The reference clinical formulation at 4% API showed substantially lower aqueous concentrations at 11 µg/ml. The higher API concentrations in a readily available micellar form in the solution and suspension formulations suggested better absorption in vivo relative to the reference clinical formulation. The lead solution (2%), lead suspension (8%) and reference clinical formulation (4%) were then administered to cynomolgus monkeys and the results were dose normalized for comparison purposes. The solution formulation demonstrated a significant improvement in exposure with a Cmax of 1750 ng/ml, compared to the reference clinical formulation Cmax of 450 ng/ml and an increase in exposure was also shown with the suspension formulation (Cmax of 650 ng/ml).
Conclusion: Solution and suspension based SEDDS formulations consisting of long-chain lipids and cosurfactant/surfactant were developed to support API solubilization in the GI. Formulation performance was assessed in vitro, against the reference clinical formulation and the solution and suspension formulations demonstrated increased drug solubilisation in the aqueous phase during lipolysis. A significant increase in exposure was demonstrated in vivo using the solution based SEDDS formulation, demonstrating bioavailabiltiy enchancement using lipid based drug delivery.
References: 1. Stegemann, S et al. Self-Emulsifying Drug Delivery Systems: Fancing the bioavailability challenge in drug delviery. Pharmazeutische Industrie. 2009. 71(8). 1409-1416.
2. Williams, H et al. Toward the establishment of standardized in vitro tests for lipid-based formulations, part 1: Method parameterization and comparison of in vitro digestion profiles across a range of representative formulations. J. Pharm. Sci.

Figure 1 Solubilized compound concentration in the aqueous phase during in vitro digestion testing of a 2 % API solution formulation (purple) and 8 % API suspension formulations (pink and green) against the 4% clinical formulation (black).

Figure 2: Plasma concentration following in vivo administration to cynomolgus monkeys of a 2 % API solution formulation (grey) and 8 % API suspension formulation (orange) against the 4% clinical formulation (blue), dose normailised.
