Back
Purpose: Dyslipidemia is recognized to be an important contributor to the progression of diabetic nephropathy (DN), leading to lipoprotein dysregulation, excessive mesangium expansion as well as inflammation in the glomeruli. Thus, dual targeting of abnormal cholesterol metabolism and inflammatory responses of mesangial cells represents an alternative approach for DN treatment. Our strategy for the treatment of DN is to develop a novel synthetic high-density lipoprotein (sHDL) nanoplatform containing 1) A kidney-targeting (KT) peptide on the surface to avoid the glomerular filtration barrier and promote mesangium retention, and 2) A Liver X Receptor (LXR) agonist in the hydrophobic core to promote cholesterol efflux in the glomeruli. This promising approach to deliver LXR agonists with sHDL nanodiscs is expected to support the idea that reducing cholesterol accumulation and inflammation in the glomeruli represents a new therapeutic approach for DN treatment.
Methods: sHDL nanodiscs were prepared by dissolving 22A peptide, DMPC, and T0901317 in acetic acid and freeze drying for 24 hours. The resulting sHDL powder was then rehydrated in PBS and reacted with lipid-conjugated KT peptide to form the kidney-targeted nanodiscs. Nanodiscs were characterized for homogeneity and purity using SEC and LCMS. Particle size was determined using DLS and negative stain TEM.Cellular uptake of nanodiscs was monitored in SV40 MES13 murine mesangial cells using sHDL nanodiscs loaded with the fluorescent dye DiO. Cholesterol efflux in mesangial cells was determined by loading cells with tritium-labeled cholesterol and measuring radioactivity counts in media and cell lysates following a 24-hour treatment period. Changes in mRNA and protein expression for downstream targets of LXR agonists were determined via RT-qPCR and western blot. The biodistribution of nanodiscs was determined by administering nanodiscs loaded with the near-infrared dye DiR and imaging mice on an in vivo imaging system (IVIS). A mouse model of DN was established via injections of streptozotocin. Efficacy in vivo was evaluated by monitoring changes in body weight, expression of inflammatory cytokines and cholesterol efflux transporters, and PAS staining of kidney tissue.
Results: sHDL nanodiscs loaded with T0901317 and conjugated with the KT peptide (KT-sHDL/T0) showed improved cellular uptake in vitro compared to unmodified nanodiscs. Treatment of murine mesangial cells with KT-sHDL/T0 resulted in improved cholesterol efflux, increased expression of the cholesterol efflux transporters ABCA1 and ABCG1, and reduced expression of the inflammatory cytokines TNFα and IL6. KT-sHDL/T0 nanodiscs showed superior accumulation in the kidneys compared to unmodified nanodiscs. KT-sHDL/T0 nanodiscs suppressed mesangial expansion, increased expression of cholesterol efflux transporters, and reduced expression of inflammatory cytokines in vivo. KT-sHDL/T0 nanodiscs demonstrated enhanced safety in vivo compared to unmodified sHDL as indicated by reduced levels of triglycerides and reduced expression of Srebp1 and Fasn
Conclusion: In this study, we developed a two-pronged strategy that was capable of meliorating dyslipidemia and inflammation in DN. The results support a potential role for LXR agonists in mitigating the progression of DN through several mechanisms, including reduction of circulating plasma lipids, inhibiting of glomerular lipid accumulation and suppression of inflammatory responses. Moreover, synthetic HDL serves as cholesterol acceptor and has a clear synergistic role with LXR ligands in DN treatment. In addition, the proven clinical safety for sHDL will likely facilitate its clinical translation as a nanocarrier. The future development of precise delivery of LXR agonists by sHDL would be beneficial in different clinical settings while reducing liver accumulation.
References: G. Russo, P. Piscitelli, A. Giandalia, F. Viazzi, R. Pontremoli, P. Fioretto, S. De Cosmo, J. Nephrol., 33 (2020), pp. 1001-1008; T. Toyama, M. Shimizu, K. Furuichi, S. Kaneko, T. Wada
Clin. Exp. Nephrol., 18 (2014), pp. 201-205
G.L. Bidwell 3rd, F. Mahdi, Q. Shao, O.C. Logue, J.P. Waller, C. Reese, A.R. Chade; Am. J. Physiol. Ren. Physiol., 312 (2017), pp. F54-f64
G. Wang, Q. Li, D. Chen, B. Wu, Y. Wu, W. Tong, P. Huang; Theranostics, 9 (2019), pp. 6191-6208
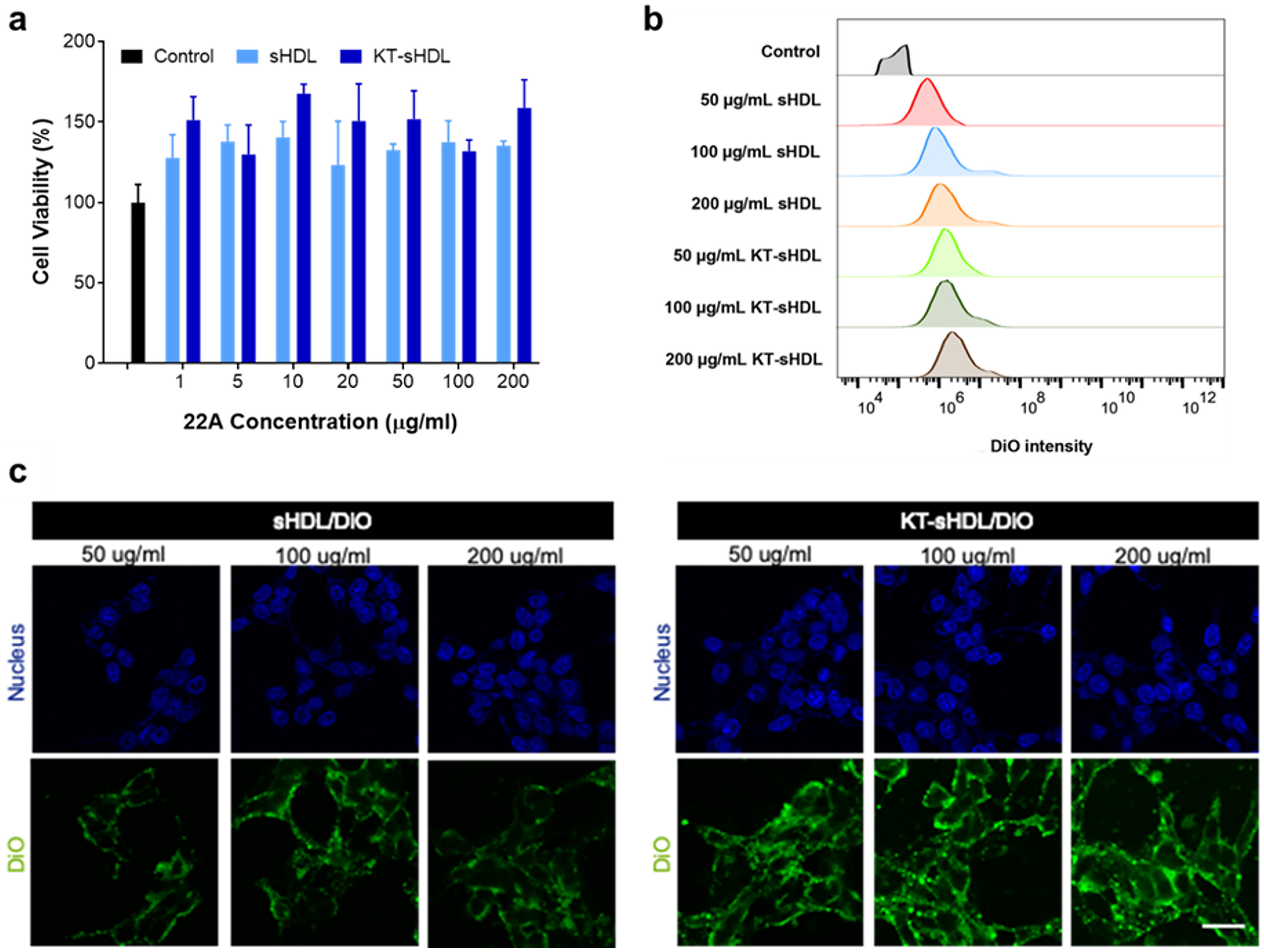
In vitro cytotoxicity of sHDL and KT-sHDL at different concentrations of 22A peptide in mesangial cells was measured by MTT assay relative to PBS control (a). Alterations in the cellular uptake were characterized by flow cytometry (b). Confocal laser scanning microscopy imaging of sHDL/DiO and KT-sHDL/DiO at different concentrations were observed for 2 h incubation at 37 °C (c).
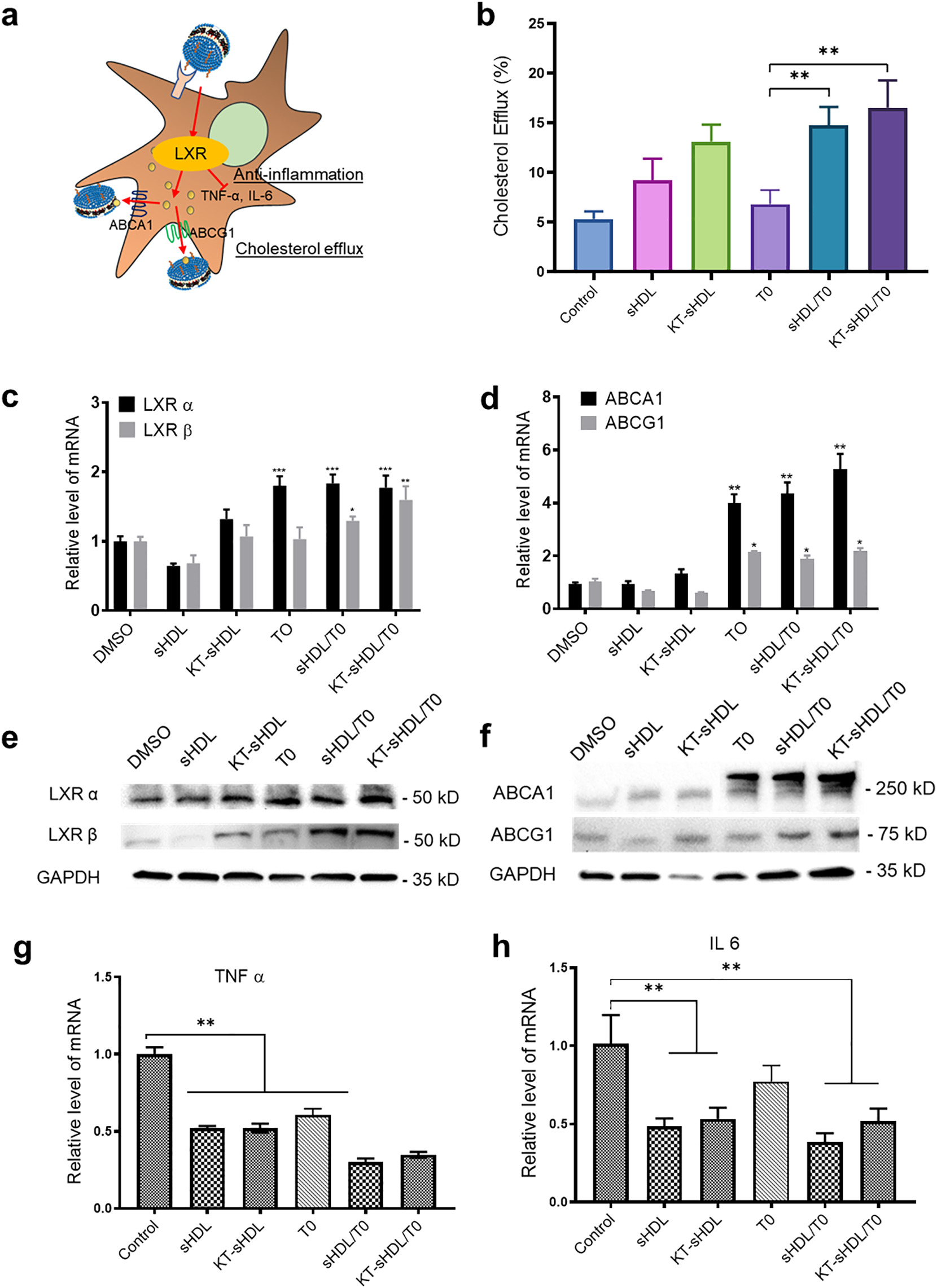
Proposed LXR agonism by KT-sHDLTO on mesangial cell (a). Cholesterol efflux from SV40 MES13 cells was measured as the percentage of [3H]-cholesterol in the medium after 12 h of incubation with free TO (0.1 μM) or different sHDL formulations (10 μg/mL 22A peptide with and without 0.1 μM TO) **p < 0.01 compared to free TO group (b). The expression of LXRα, LXRβ, ABCA1 and ABCG1 were measured by Western blot after 24 h treatment with different formulations(c). RT-PCR was performed for LXR α, LXR β, ABCA1 and ABCG1, and quantification was performed using the ddCt method normalizing to DMSO control, *p < 0.5, **p < 0.01, ***p < 0.001 compared to DMSO control (e, f). SV40 MES13 cell were pretreated with high glucose for 24 h and then incubated with different sHDL formulations for 48 h. Expression of proinflammatory cytokines was determined in each group by RT-qPCR (g,h), **p < 0.01 compared to DMSO control. Data are means ± SD; n = 3–5 per group.
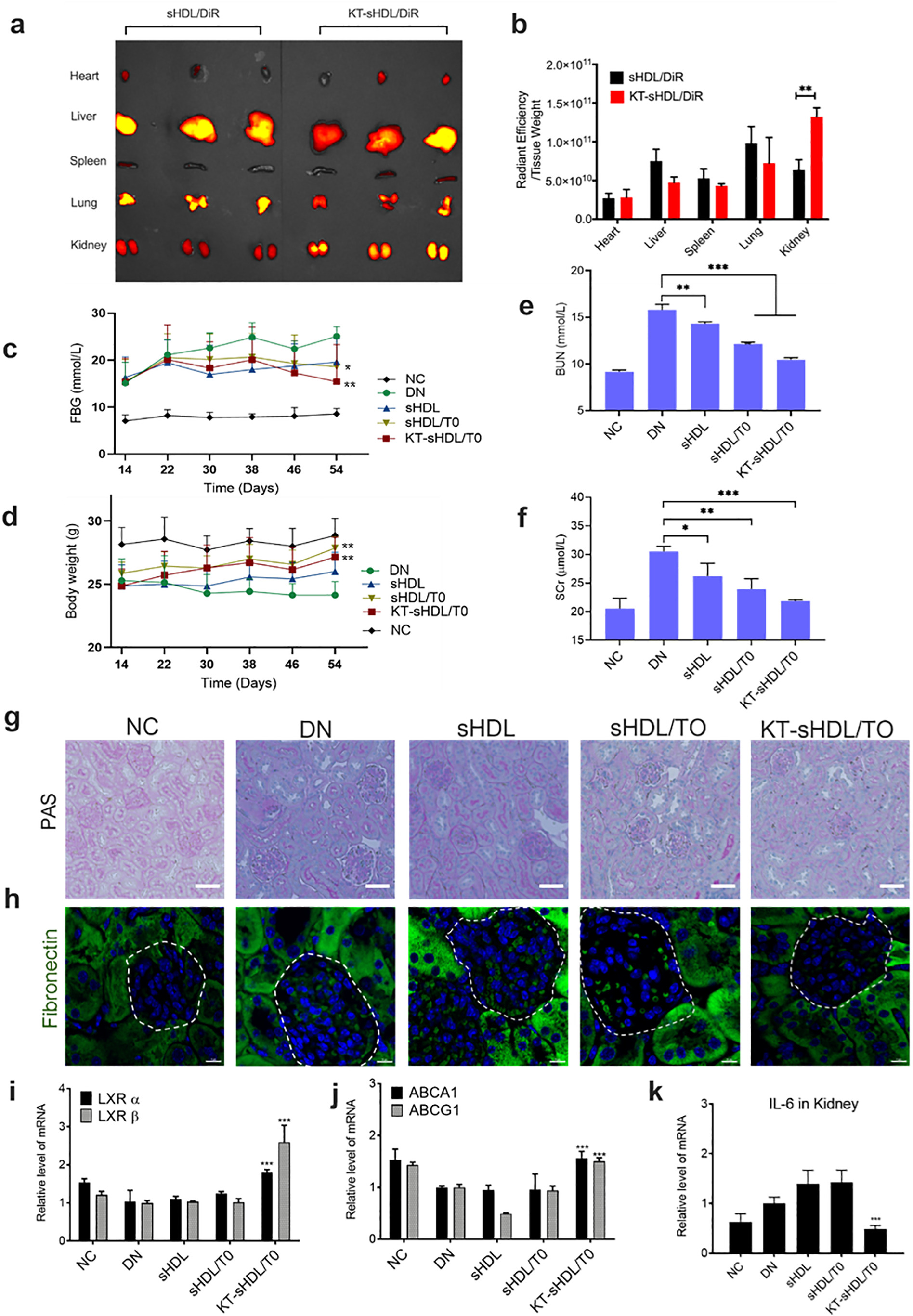
After 12 h I.V. injection of sHDL/DiR and KT-sHDL/DiR in healthy mice, major organs were extracted and images were obtained using IVIS (a). The quantitative analysis of fluorescence intensity in each organ was indicated as the unit of radiant efficiency by using the Living Image software (b). **p < 0.01. Changes in FBG (c) and body weight (d) were displayed in each group during treatment in diseased mice under diabetic condition. At the end of the treatment period, blood urea nitrogen (BUN) and serum creatinine (SCr) of the mice in each group were measured and analyzed (e, f). Representative photographs of glomerular mesangial expansion in mice after different treatment was imaged by PAS staining, scale bar = 50 μm (g). Endogenous fibronectin (FN) expression was detected by immunofluorescent staining for FN (green) and nuclei (blue). Magnification, x63, Scale bar = 10 μm (h). The expression of LXR receptors (i), cholesterol efflux transporters (j) and proinflammatory cytokines (k) were evaluated by RT-PCR analysis. Data were presented as Mean ± SD. Comparison with DN control group, *p < 0.05, **p < 0.01, *** p < 0.005.
Formulation and Delivery - Chemical - Drug Delivery
Category: Late Breaking Poster Abstract
(M1130-07-39) Nanodisc Delivery of Liver X Receptor Agonist for the Treatment of Diabetic Nephropathy
Monday, October 17, 2022
11:30 AM – 12:30 PM ET
- TH
Troy Halseth
University of Michigan
Ann Arbor, Michigan, United States - HH
Hongliang He, Ph.D.
Southeast University
Nanjing, Jiangsu, China (People's Republic)
Presenting Author(s)
Main Author(s)
Purpose: Dyslipidemia is recognized to be an important contributor to the progression of diabetic nephropathy (DN), leading to lipoprotein dysregulation, excessive mesangium expansion as well as inflammation in the glomeruli. Thus, dual targeting of abnormal cholesterol metabolism and inflammatory responses of mesangial cells represents an alternative approach for DN treatment. Our strategy for the treatment of DN is to develop a novel synthetic high-density lipoprotein (sHDL) nanoplatform containing 1) A kidney-targeting (KT) peptide on the surface to avoid the glomerular filtration barrier and promote mesangium retention, and 2) A Liver X Receptor (LXR) agonist in the hydrophobic core to promote cholesterol efflux in the glomeruli. This promising approach to deliver LXR agonists with sHDL nanodiscs is expected to support the idea that reducing cholesterol accumulation and inflammation in the glomeruli represents a new therapeutic approach for DN treatment.
Methods: sHDL nanodiscs were prepared by dissolving 22A peptide, DMPC, and T0901317 in acetic acid and freeze drying for 24 hours. The resulting sHDL powder was then rehydrated in PBS and reacted with lipid-conjugated KT peptide to form the kidney-targeted nanodiscs. Nanodiscs were characterized for homogeneity and purity using SEC and LCMS. Particle size was determined using DLS and negative stain TEM.Cellular uptake of nanodiscs was monitored in SV40 MES13 murine mesangial cells using sHDL nanodiscs loaded with the fluorescent dye DiO. Cholesterol efflux in mesangial cells was determined by loading cells with tritium-labeled cholesterol and measuring radioactivity counts in media and cell lysates following a 24-hour treatment period. Changes in mRNA and protein expression for downstream targets of LXR agonists were determined via RT-qPCR and western blot. The biodistribution of nanodiscs was determined by administering nanodiscs loaded with the near-infrared dye DiR and imaging mice on an in vivo imaging system (IVIS). A mouse model of DN was established via injections of streptozotocin. Efficacy in vivo was evaluated by monitoring changes in body weight, expression of inflammatory cytokines and cholesterol efflux transporters, and PAS staining of kidney tissue.
Results: sHDL nanodiscs loaded with T0901317 and conjugated with the KT peptide (KT-sHDL/T0) showed improved cellular uptake in vitro compared to unmodified nanodiscs. Treatment of murine mesangial cells with KT-sHDL/T0 resulted in improved cholesterol efflux, increased expression of the cholesterol efflux transporters ABCA1 and ABCG1, and reduced expression of the inflammatory cytokines TNFα and IL6. KT-sHDL/T0 nanodiscs showed superior accumulation in the kidneys compared to unmodified nanodiscs. KT-sHDL/T0 nanodiscs suppressed mesangial expansion, increased expression of cholesterol efflux transporters, and reduced expression of inflammatory cytokines in vivo. KT-sHDL/T0 nanodiscs demonstrated enhanced safety in vivo compared to unmodified sHDL as indicated by reduced levels of triglycerides and reduced expression of Srebp1 and Fasn
Conclusion: In this study, we developed a two-pronged strategy that was capable of meliorating dyslipidemia and inflammation in DN. The results support a potential role for LXR agonists in mitigating the progression of DN through several mechanisms, including reduction of circulating plasma lipids, inhibiting of glomerular lipid accumulation and suppression of inflammatory responses. Moreover, synthetic HDL serves as cholesterol acceptor and has a clear synergistic role with LXR ligands in DN treatment. In addition, the proven clinical safety for sHDL will likely facilitate its clinical translation as a nanocarrier. The future development of precise delivery of LXR agonists by sHDL would be beneficial in different clinical settings while reducing liver accumulation.
References: G. Russo, P. Piscitelli, A. Giandalia, F. Viazzi, R. Pontremoli, P. Fioretto, S. De Cosmo, J. Nephrol., 33 (2020), pp. 1001-1008; T. Toyama, M. Shimizu, K. Furuichi, S. Kaneko, T. Wada
Clin. Exp. Nephrol., 18 (2014), pp. 201-205
G.L. Bidwell 3rd, F. Mahdi, Q. Shao, O.C. Logue, J.P. Waller, C. Reese, A.R. Chade; Am. J. Physiol. Ren. Physiol., 312 (2017), pp. F54-f64
G. Wang, Q. Li, D. Chen, B. Wu, Y. Wu, W. Tong, P. Huang; Theranostics, 9 (2019), pp. 6191-6208

In vitro cytotoxicity of sHDL and KT-sHDL at different concentrations of 22A peptide in mesangial cells was measured by MTT assay relative to PBS control (a). Alterations in the cellular uptake were characterized by flow cytometry (b). Confocal laser scanning microscopy imaging of sHDL/DiO and KT-sHDL/DiO at different concentrations were observed for 2 h incubation at 37 °C (c).

Proposed LXR agonism by KT-sHDLTO on mesangial cell (a). Cholesterol efflux from SV40 MES13 cells was measured as the percentage of [3H]-cholesterol in the medium after 12 h of incubation with free TO (0.1 μM) or different sHDL formulations (10 μg/mL 22A peptide with and without 0.1 μM TO) **p < 0.01 compared to free TO group (b). The expression of LXRα, LXRβ, ABCA1 and ABCG1 were measured by Western blot after 24 h treatment with different formulations(c). RT-PCR was performed for LXR α, LXR β, ABCA1 and ABCG1, and quantification was performed using the ddCt method normalizing to DMSO control, *p < 0.5, **p < 0.01, ***p < 0.001 compared to DMSO control (e, f). SV40 MES13 cell were pretreated with high glucose for 24 h and then incubated with different sHDL formulations for 48 h. Expression of proinflammatory cytokines was determined in each group by RT-qPCR (g,h), **p < 0.01 compared to DMSO control. Data are means ± SD; n = 3–5 per group.

After 12 h I.V. injection of sHDL/DiR and KT-sHDL/DiR in healthy mice, major organs were extracted and images were obtained using IVIS (a). The quantitative analysis of fluorescence intensity in each organ was indicated as the unit of radiant efficiency by using the Living Image software (b). **p < 0.01. Changes in FBG (c) and body weight (d) were displayed in each group during treatment in diseased mice under diabetic condition. At the end of the treatment period, blood urea nitrogen (BUN) and serum creatinine (SCr) of the mice in each group were measured and analyzed (e, f). Representative photographs of glomerular mesangial expansion in mice after different treatment was imaged by PAS staining, scale bar = 50 μm (g). Endogenous fibronectin (FN) expression was detected by immunofluorescent staining for FN (green) and nuclei (blue). Magnification, x63, Scale bar = 10 μm (h). The expression of LXR receptors (i), cholesterol efflux transporters (j) and proinflammatory cytokines (k) were evaluated by RT-PCR analysis. Data were presented as Mean ± SD. Comparison with DN control group, *p < 0.05, **p < 0.01, *** p < 0.005.
