Back
Purpose: Oral mucositis is a common side effect of cancer therapy which is accompanied by uncontrolled pain. Current therapies for pain management include a lidocaine liquid oral rinse and opioids. However, these strategies have limitations. Lidocaine oral rinse exhibits a short duration of pain relief (less than 30 min) due to the short half-life of lidocaine and the short contact time between the liquid formulation and the oral mucosa. Though opioids are powerful analgesics, their long-term use is limited due to their addictive potential, as well as side effects such as somnolence and constipation. To overcome the limitations of existing therapies, a mucoadhesive in situ forming gel containing Bupivacaine HCl is proposed to achieve a prolonged duration of pain control. The formulation can be selectively sprayed on the affected mucosal area to minimize side effects and it contains mucoadhesive polymers to enhance the mucosal contact time. Bupivacaine HCl was used as the model drug since it has a high lipid solubility, which favors drug diffusion through the lipid-rich neural membranes of the axons resulting in high anesthetic efficacy.
Methods: Two formulations containing Bupivacaine HCl were prepared using a marketed Bupivacaine HCl solution for injection, and two different gelling agents. Each formulation had a different mucoadhesive polymer (polymer 1 or polymer 2). The formulations and their preparation methods were optimized to achieve adequate gelation and mucoadhesion. The formulations were characterized for drug loading using HPLC, gelation temperature via tube inversion method, ex vivo mucoadhesion using porcine buccal mucosa via a texture analyzer, in vitro drug release using USP II apparatus equipped with enhancer cells, and sprayability. Short-term stability was investigated under 2-8 ⁰C and room temperature for up to 2 weeks. Rheological analysis of the thermosensitive behavior of the in situ forming gels was carried out using a cone and plate viscometer.
Results: It was observed that mucoadhesive polymer 1 did not interfere with the in situ gelling behavior of the formulation, showed excellent mucoadhesion, and had high drug release rates. However, these formulations appeared slightly hazy. On the other hand, formulations containing mucoadhesive polymer 2 increased the gelling temperature, showed slow drug release, and formed clear homogeneous solutions. The release kinetics of the formulations followed both the Higuchi and the first-order models. Increase in the concentration of mucoadhesive polymer 2 led to higher mucoadhesion and viscosity while compromising the sprayability of the formulations. Optimum mucoadhesion, gelation, and sprayability were achieved using a lower concentration of mucoadhesive polymer 2. Short-term stability studies revealed that the viscosities of the formulations increased at 2-8 ⁰C which negatively impacted the sprayability. Accordingly, room temperature is recommended for short-term storage of these formulations. The gelling behavior of the formulations studied via rheological analysis corroborated the observed gelling temperature via the tube inversion method. DSC and FTIR analysis revealed the characteristic peaks attributed to the drug and the excipients, indicating no significant drug-excipient interactions.
Conclusion: Selection of the type and concentration of excipients is critical to achieving the desired attributes of in situ forming gels. The optimized formulation containing mucoadhesive polymer 2 showed good in vitro performance and phase 1 clinical trials are planned to start shortly. The optimized formulation was suitable for short-term storage at room temperature prior to use. A patent application is pending to protect the intellectual property developed during this work.
References: 1. Li T., et.al. Mucoadhesive in situ forming gel for oral mucositis pain control. Int. J. Pharm., 2020. 580, 119238.
2. Li T., et al. Enhanced drug loading of in situ forming gels for oral mucositis pain control. Int. J. Pharm., 2021. 595, 120225.
Acknowledgements: This work was supported by the University of Connecticut, Pfizer Distinguished Chair in Pharmaceutical Technology funds, UConn SPARK Technology Commercialization Fund, and the International Association for Dental Research (Innovation in Oral Care award fund).
Disclaimer: A patent application is pending to protect the intellectual property developed during this work.
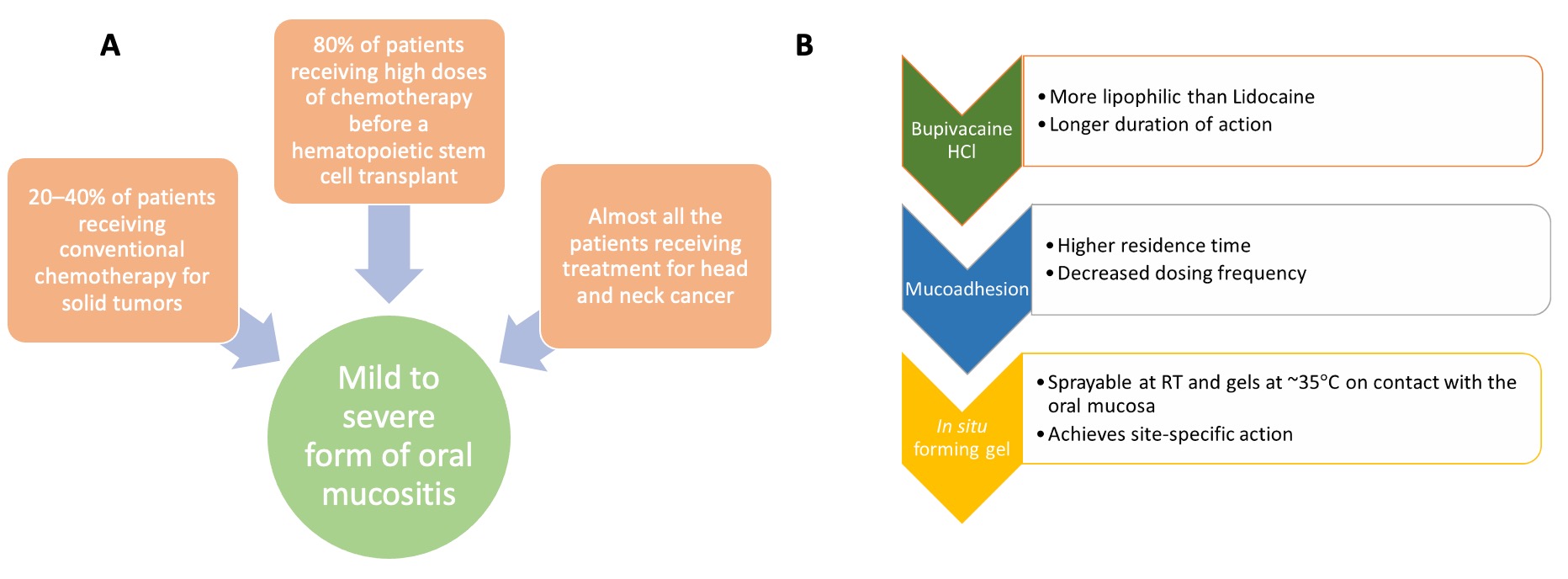
Fig. 1. A) Demographics of oral mucositis; B) rationale for the development of oral mucoadhesive in situ forming gel.
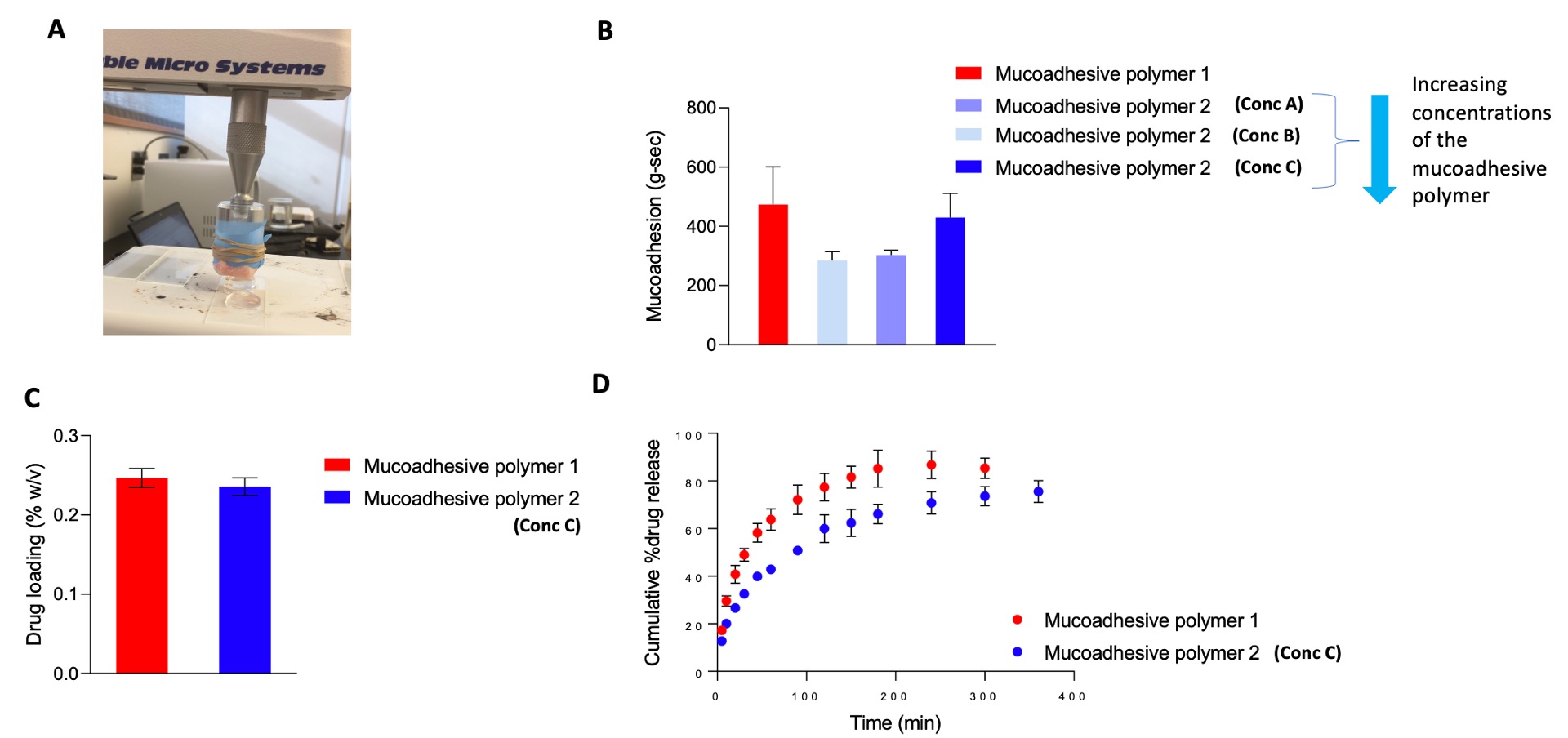
Fig. 2. A) Assembly used for ex vivo mucoadhesion studies with excised porcine buccal mucosa; B) mucoadhesion (g.sec) of the formulations with different mucoadhesive polymers via a texture analyzer (mean ±SD, n=3); C) drug loading of the optimized formulations (mean ±SD, n=3); D) in vitro release profiles of the formulations in artificial saliva pH 6.8 (mean ±SD, n=3).
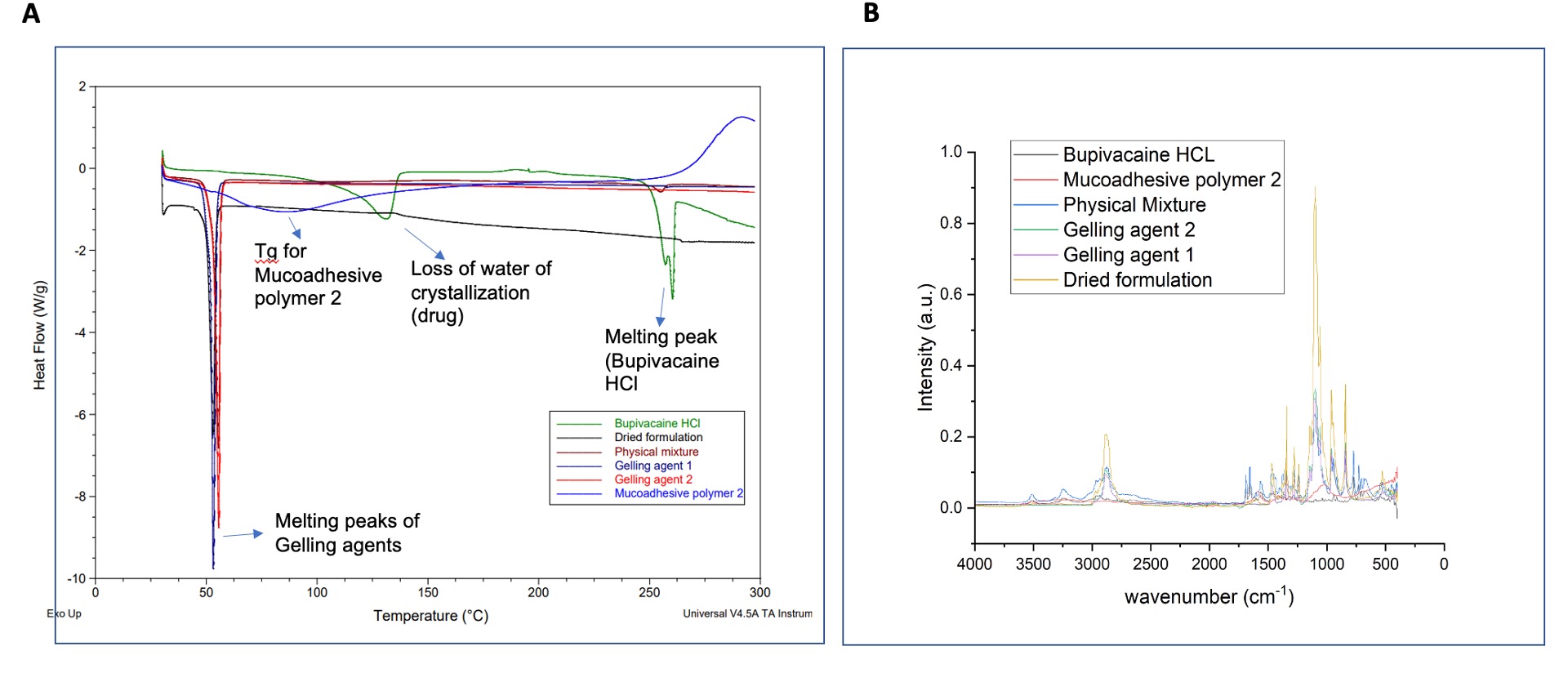
Fig. 3. A) DSC thermograms of drug (Bupivacaine HCl), excipients, and formulation; B) FTIR spectra of drug, excipients, and formulation.
Formulation and Delivery - Chemical - Drug Delivery
Category: Poster Abstract
(W1130-03-15) Development of Bupivacaine Hydrochloride Mucoadhesive In Situ Forming Gels for Oral Mucositis
Wednesday, October 19, 2022
11:30 AM – 12:30 PM ET
- RK
Radha Kulkarni, MS
University of Connecticut
Willimantic, Connecticut, United States - RK
Radha Kulkarni, MS
University of Connecticut
Willimantic, Connecticut, United States
Presenting Author(s)
Main Author(s)
Purpose: Oral mucositis is a common side effect of cancer therapy which is accompanied by uncontrolled pain. Current therapies for pain management include a lidocaine liquid oral rinse and opioids. However, these strategies have limitations. Lidocaine oral rinse exhibits a short duration of pain relief (less than 30 min) due to the short half-life of lidocaine and the short contact time between the liquid formulation and the oral mucosa. Though opioids are powerful analgesics, their long-term use is limited due to their addictive potential, as well as side effects such as somnolence and constipation. To overcome the limitations of existing therapies, a mucoadhesive in situ forming gel containing Bupivacaine HCl is proposed to achieve a prolonged duration of pain control. The formulation can be selectively sprayed on the affected mucosal area to minimize side effects and it contains mucoadhesive polymers to enhance the mucosal contact time. Bupivacaine HCl was used as the model drug since it has a high lipid solubility, which favors drug diffusion through the lipid-rich neural membranes of the axons resulting in high anesthetic efficacy.
Methods: Two formulations containing Bupivacaine HCl were prepared using a marketed Bupivacaine HCl solution for injection, and two different gelling agents. Each formulation had a different mucoadhesive polymer (polymer 1 or polymer 2). The formulations and their preparation methods were optimized to achieve adequate gelation and mucoadhesion. The formulations were characterized for drug loading using HPLC, gelation temperature via tube inversion method, ex vivo mucoadhesion using porcine buccal mucosa via a texture analyzer, in vitro drug release using USP II apparatus equipped with enhancer cells, and sprayability. Short-term stability was investigated under 2-8 ⁰C and room temperature for up to 2 weeks. Rheological analysis of the thermosensitive behavior of the in situ forming gels was carried out using a cone and plate viscometer.
Results: It was observed that mucoadhesive polymer 1 did not interfere with the in situ gelling behavior of the formulation, showed excellent mucoadhesion, and had high drug release rates. However, these formulations appeared slightly hazy. On the other hand, formulations containing mucoadhesive polymer 2 increased the gelling temperature, showed slow drug release, and formed clear homogeneous solutions. The release kinetics of the formulations followed both the Higuchi and the first-order models. Increase in the concentration of mucoadhesive polymer 2 led to higher mucoadhesion and viscosity while compromising the sprayability of the formulations. Optimum mucoadhesion, gelation, and sprayability were achieved using a lower concentration of mucoadhesive polymer 2. Short-term stability studies revealed that the viscosities of the formulations increased at 2-8 ⁰C which negatively impacted the sprayability. Accordingly, room temperature is recommended for short-term storage of these formulations. The gelling behavior of the formulations studied via rheological analysis corroborated the observed gelling temperature via the tube inversion method. DSC and FTIR analysis revealed the characteristic peaks attributed to the drug and the excipients, indicating no significant drug-excipient interactions.
Conclusion: Selection of the type and concentration of excipients is critical to achieving the desired attributes of in situ forming gels. The optimized formulation containing mucoadhesive polymer 2 showed good in vitro performance and phase 1 clinical trials are planned to start shortly. The optimized formulation was suitable for short-term storage at room temperature prior to use. A patent application is pending to protect the intellectual property developed during this work.
References: 1. Li T., et.al. Mucoadhesive in situ forming gel for oral mucositis pain control. Int. J. Pharm., 2020. 580, 119238.
2. Li T., et al. Enhanced drug loading of in situ forming gels for oral mucositis pain control. Int. J. Pharm., 2021. 595, 120225.
Acknowledgements: This work was supported by the University of Connecticut, Pfizer Distinguished Chair in Pharmaceutical Technology funds, UConn SPARK Technology Commercialization Fund, and the International Association for Dental Research (Innovation in Oral Care award fund).
Disclaimer: A patent application is pending to protect the intellectual property developed during this work.

Fig. 1. A) Demographics of oral mucositis; B) rationale for the development of oral mucoadhesive in situ forming gel.

Fig. 2. A) Assembly used for ex vivo mucoadhesion studies with excised porcine buccal mucosa; B) mucoadhesion (g.sec) of the formulations with different mucoadhesive polymers via a texture analyzer (mean ±SD, n=3); C) drug loading of the optimized formulations (mean ±SD, n=3); D) in vitro release profiles of the formulations in artificial saliva pH 6.8 (mean ±SD, n=3).

Fig. 3. A) DSC thermograms of drug (Bupivacaine HCl), excipients, and formulation; B) FTIR spectra of drug, excipients, and formulation.
