Back
Purpose: The purpose of this study is to prepare and evaluate homogeneous liposomal nanoparticles through high press homogenizer by novel protein prodrug (MET 0226) mixing with lecithin dispersion after precipitating poorly soluble protein. Protein pro-drug (MET 0226) in this experiment is novel linear protein that does not require maintenance of a tertiary structure during the formulation. because the protein prodrug (MET 0226) is activated and folded to tertiary structure in the intracellular system after delivery into the cell. Liposomal nanoparticles are the most suitable drug delivery system to solve this protein’s poorly soluble property and intercellular delivery.
Methods: MET 0226 protein in pH 3.5 8M urea solution was titrated by adding 1N NaOH solution for the first precipitation process targeted in the pH7, pH9, and pH11. For the second precipitation process, ethanol was added with MET 0226 protein in denaturation solvent: EtOH = 1: 5 (v/v), and the precipitation yield was confirmed using NITM (Non-Interfering) protein assay (G-Biosciences). The precipitated MET 0226 protein was centrifuged using Microcentrifuge (LABOGENE 1730R) at 5927xg, 4°C for 15mins and mixed with 1%(w/v) egg lecithin (Lipoid E 80) dispersion. MET 0226 protein mixed with 1%(w/v) egg lecithin was homogenized using a high press homogenizer (HPH, Nano DeBEE homogenizer, BEE international Co.) to prepare MET 0226 protein loaded liposomal nanoparticles. In the process of HPH, pressure was compared for 5000 psi, 10000 psi, and 15000 psi, and the number of cycles was compared for 1cycle, 2 cycles and 3 cycles. Process parameter screening for pressure and number of cycles was compared to evaluate in particle distribution and Poly diversity index was evaluated through dynamic light scattering (DLS, ELS-Z, Otsuka Electronics). The morphology of MET 0226 loaded liposomal nanoparticles was evaluated by scanning electron microscopy (SEM, JSM-7800F) and transmission electron microscopy (TEM, JEM-F200). Stabilizers Glycerol, D-sorbitol, D-mannitol, ascorbic acid and alpha-tocopherol polyethylene glycol 1000 succinate (Vit E-TPGS) were added to the liposomal nanoparticles, respectively, and the stability test was carried out for 21 days and evaluated by screening the particle size distribution using the DLS.
Results: Preparation MET 0226 protein pro-drug loaded liposomal nanoparticles depend on process parameter screening. Figure 1 A shows the graph of pH titration while adding 1N NaOH solution to the MET 0226 8M Urea pH 3.5 denatured solvent to determine the isoelectric point of MET 0226.
At pH 7 to 9, protein precipitation occurred, and at pH 11 it became solubilized again. Thereafter, MET 0226 protein: EtOH = 1: 5 (v/v) was added for secondary precipitation to maximize protein precipitation. Figure 1 B shows the particle size, distribution, and filtering yield according to the pressure and number of cycles of the high press homogenizer. From 5000 psi to 15000 psi, the average particle size is less than 200 nm, and when filtered, it had smaller and showed a sharp single particle distribution. When comparing the filtering efficiency according to the pressure and number of cycles of the high press homogenizer using a 0.2um cellulose acetate syringe filter, the highest yield 84.5% was obtained at 15000 psi 3 cycles. On the other hand, 5000 psi and 10000 psi had a yield of less than 35%. Morphology of MET 0226 protein pro-drug loaded liposomal nanoparticle Figure 1C shows the SEM and TEM observation of the morphology of the MET 0226 loaded liposomal nanoparticle. When The MET 0226 liposomal nanoparticle was observed after freeze-drying, it showed agglomerated form. Stability test
Figure 1D shows a graph of particle size, PDI, and D (90%) measured over time after different stabilizers were added to the liposomal nanoparticle. After 21 days, the particle size of all formulations was maintained below 200 nm without significant change, and PDI was also maintained below 0.31. On the other hand, in all formulations, the D (90%) value commonly showed a tendency to increase more on the Day 21 compared with the Day 1. Among them, the formulation in which VitE-TPGS was added as a stabilizer showed the smallest D (90%) value of less than about 400 nm.
Conclusion: This study presents the steps for establishing a process to prepare liposomal nanoparticle containing a poorly soluble protein called MET 0226. When protein pellets are mixed with 1% (w/v) lecithin dispersion and process parameters for pressure and number of cycles are compared in the process of High Press Homogenizer (HPH), the particle size is 200 nm or less when manufactured under the conditions of 15000 psi and 3 cycles. High protein yield (%) after 0.2um filtration and uniform particle distribution were obtained. Formation of liposomal nanoparticles was confirmed by confirming the morphological characteristics of the formulation by SEM and TEM. When the stability of liposomal nanoparticles was compared by adding various stabilizers to enhance the stability of the liposomal nanoparticles, the particle size was stably maintained under 200nm at 4°C storage condition for up to 21 days.
References: Kim, S., Jeong, C. H., Song, S. H., Um, J. E., Kim, H. S., Yun, J. S., ... & Yoo, T. H. (2020). Micellized Protein Transduction Domain-Bone Morphogenetic Protein-7 Efficiently Blocks Renal Fibrosis Via Inhibition of Transforming Growth Factor-Beta–Mediated Epithelial–Mesenchymal Transition. Frontiers in Pharmacology, 11, 1769.
Acknowledgements:
Funding: This work was supported by the Mid-Career Researcher Program (No. NRF-2021R1A2C2008834) and Basic Research Infrastructure Support Program (University-Centered Labs-2018R1A6A1A03023718) through the National Research Foundation of Korea (NRF) funded by the Korean government (MSIT)
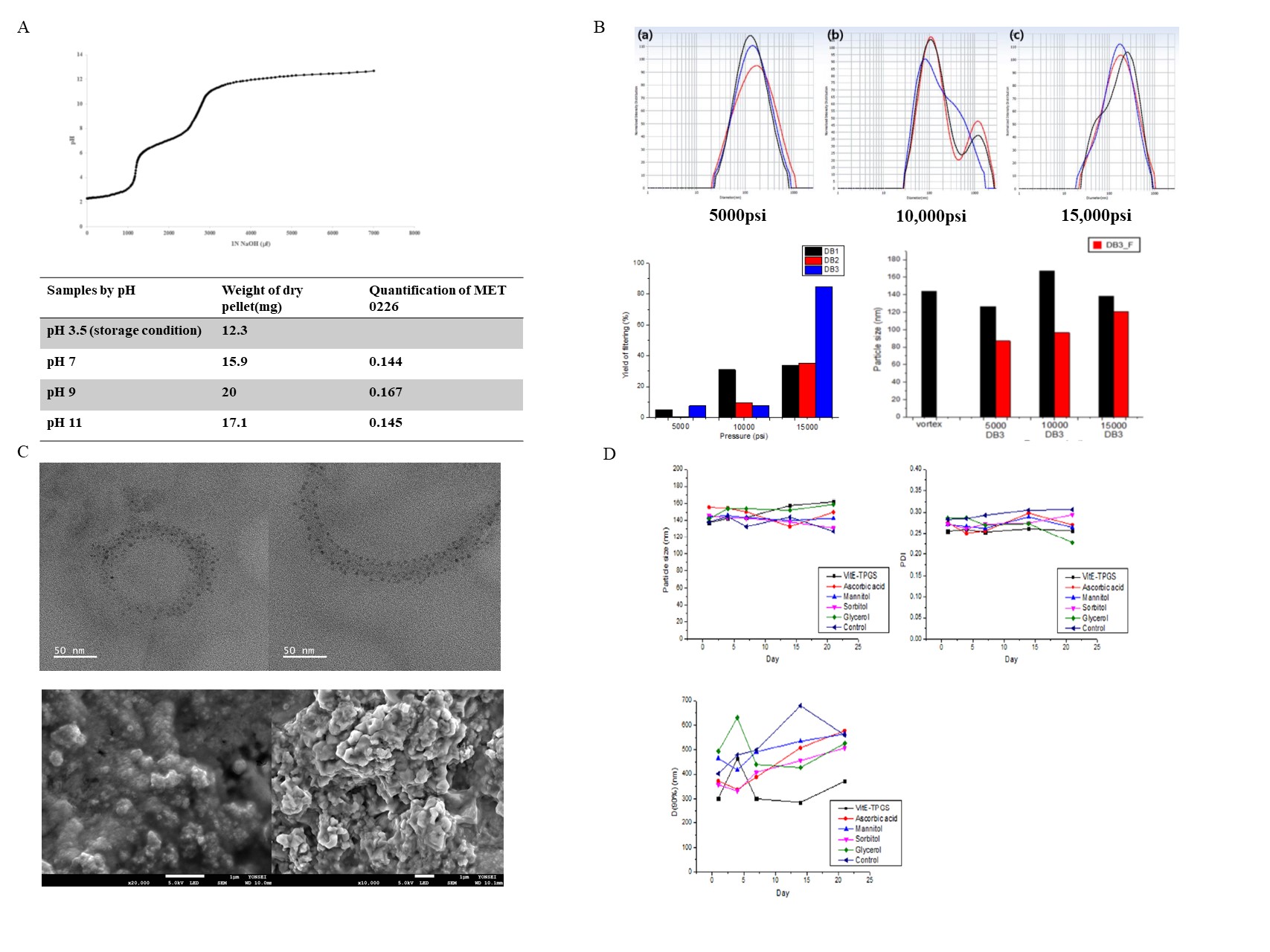
Figure 1. (A) MET 0226 protein titration curve, quantification and weight of protein dry pellet by pH. (B) Different particle sizes and yields with pressure and number of cycles of MET 0226 loaded liposomal nanoparticles. (C) Morphology of TEM(up) and SEM(down) of MET 0226 loaded liposomal nanoparticles. (D) Particle size and distribution changes according to the stabilizer of MET 0226 loaded liposomal nanoparticles.
Formulation and Delivery - Biomolecular - Formulation
Category: Poster Abstract
(M1330-04-21) Preparation and Characterization of Poorly Soluble Protein (MET 0226) Liposomal Nanoparticle by High Press Homogenizer
Monday, October 17, 2022
1:30 PM – 2:30 PM ET
- JL
Jong-Ju Lee, Pharm.D.
Yonsei University
Incheon, Inch'on-jikhalsi, Republic of Korea - JL
Jong-Ju Lee, Pharm.D.
Yonsei University
Incheon, Inch'on-jikhalsi, Republic of Korea
Presenting Author(s)
Main Author(s)
Purpose: The purpose of this study is to prepare and evaluate homogeneous liposomal nanoparticles through high press homogenizer by novel protein prodrug (MET 0226) mixing with lecithin dispersion after precipitating poorly soluble protein. Protein pro-drug (MET 0226) in this experiment is novel linear protein that does not require maintenance of a tertiary structure during the formulation. because the protein prodrug (MET 0226) is activated and folded to tertiary structure in the intracellular system after delivery into the cell. Liposomal nanoparticles are the most suitable drug delivery system to solve this protein’s poorly soluble property and intercellular delivery.
Methods: MET 0226 protein in pH 3.5 8M urea solution was titrated by adding 1N NaOH solution for the first precipitation process targeted in the pH7, pH9, and pH11. For the second precipitation process, ethanol was added with MET 0226 protein in denaturation solvent: EtOH = 1: 5 (v/v), and the precipitation yield was confirmed using NITM (Non-Interfering) protein assay (G-Biosciences). The precipitated MET 0226 protein was centrifuged using Microcentrifuge (LABOGENE 1730R) at 5927xg, 4°C for 15mins and mixed with 1%(w/v) egg lecithin (Lipoid E 80) dispersion. MET 0226 protein mixed with 1%(w/v) egg lecithin was homogenized using a high press homogenizer (HPH, Nano DeBEE homogenizer, BEE international Co.) to prepare MET 0226 protein loaded liposomal nanoparticles. In the process of HPH, pressure was compared for 5000 psi, 10000 psi, and 15000 psi, and the number of cycles was compared for 1cycle, 2 cycles and 3 cycles. Process parameter screening for pressure and number of cycles was compared to evaluate in particle distribution and Poly diversity index was evaluated through dynamic light scattering (DLS, ELS-Z, Otsuka Electronics). The morphology of MET 0226 loaded liposomal nanoparticles was evaluated by scanning electron microscopy (SEM, JSM-7800F) and transmission electron microscopy (TEM, JEM-F200). Stabilizers Glycerol, D-sorbitol, D-mannitol, ascorbic acid and alpha-tocopherol polyethylene glycol 1000 succinate (Vit E-TPGS) were added to the liposomal nanoparticles, respectively, and the stability test was carried out for 21 days and evaluated by screening the particle size distribution using the DLS.
Results: Preparation MET 0226 protein pro-drug loaded liposomal nanoparticles depend on process parameter screening. Figure 1 A shows the graph of pH titration while adding 1N NaOH solution to the MET 0226 8M Urea pH 3.5 denatured solvent to determine the isoelectric point of MET 0226.
At pH 7 to 9, protein precipitation occurred, and at pH 11 it became solubilized again. Thereafter, MET 0226 protein: EtOH = 1: 5 (v/v) was added for secondary precipitation to maximize protein precipitation. Figure 1 B shows the particle size, distribution, and filtering yield according to the pressure and number of cycles of the high press homogenizer. From 5000 psi to 15000 psi, the average particle size is less than 200 nm, and when filtered, it had smaller and showed a sharp single particle distribution. When comparing the filtering efficiency according to the pressure and number of cycles of the high press homogenizer using a 0.2um cellulose acetate syringe filter, the highest yield 84.5% was obtained at 15000 psi 3 cycles. On the other hand, 5000 psi and 10000 psi had a yield of less than 35%. Morphology of MET 0226 protein pro-drug loaded liposomal nanoparticle Figure 1C shows the SEM and TEM observation of the morphology of the MET 0226 loaded liposomal nanoparticle. When The MET 0226 liposomal nanoparticle was observed after freeze-drying, it showed agglomerated form. Stability test
Figure 1D shows a graph of particle size, PDI, and D (90%) measured over time after different stabilizers were added to the liposomal nanoparticle. After 21 days, the particle size of all formulations was maintained below 200 nm without significant change, and PDI was also maintained below 0.31. On the other hand, in all formulations, the D (90%) value commonly showed a tendency to increase more on the Day 21 compared with the Day 1. Among them, the formulation in which VitE-TPGS was added as a stabilizer showed the smallest D (90%) value of less than about 400 nm.
Conclusion: This study presents the steps for establishing a process to prepare liposomal nanoparticle containing a poorly soluble protein called MET 0226. When protein pellets are mixed with 1% (w/v) lecithin dispersion and process parameters for pressure and number of cycles are compared in the process of High Press Homogenizer (HPH), the particle size is 200 nm or less when manufactured under the conditions of 15000 psi and 3 cycles. High protein yield (%) after 0.2um filtration and uniform particle distribution were obtained. Formation of liposomal nanoparticles was confirmed by confirming the morphological characteristics of the formulation by SEM and TEM. When the stability of liposomal nanoparticles was compared by adding various stabilizers to enhance the stability of the liposomal nanoparticles, the particle size was stably maintained under 200nm at 4°C storage condition for up to 21 days.
References: Kim, S., Jeong, C. H., Song, S. H., Um, J. E., Kim, H. S., Yun, J. S., ... & Yoo, T. H. (2020). Micellized Protein Transduction Domain-Bone Morphogenetic Protein-7 Efficiently Blocks Renal Fibrosis Via Inhibition of Transforming Growth Factor-Beta–Mediated Epithelial–Mesenchymal Transition. Frontiers in Pharmacology, 11, 1769.
Acknowledgements:
Funding: This work was supported by the Mid-Career Researcher Program (No. NRF-2021R1A2C2008834) and Basic Research Infrastructure Support Program (University-Centered Labs-2018R1A6A1A03023718) through the National Research Foundation of Korea (NRF) funded by the Korean government (MSIT)

Figure 1. (A) MET 0226 protein titration curve, quantification and weight of protein dry pellet by pH. (B) Different particle sizes and yields with pressure and number of cycles of MET 0226 loaded liposomal nanoparticles. (C) Morphology of TEM(up) and SEM(down) of MET 0226 loaded liposomal nanoparticles. (D) Particle size and distribution changes according to the stabilizer of MET 0226 loaded liposomal nanoparticles.
