Back
Poster Session A
Rheumatoid arthritis (RA)
Session: (0241–0271) RA – Diagnosis, Manifestations, and Outcomes Poster I
0267: Cancer Risk with Biologic and Targeted Synthetic DMARDs in Patients with Rheumatic Diseases and Previous Malignancy: Results from the BIOBADASER Register
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall

Juan Molina-Collada, MD
Hospital General Universitario Gregorio Marañón
Madrid, Spain
Abstract Poster Presenter(s)
Juan Molina1, Fernando Sanchez-Alonso2, Cristina Bohorquez3, Cesar Diaz-Torne4, Carolina Perez-Garcia5, Juan Maria Blanco-Madrigal6, Paloma Vela-Casampere7, José María Älvaro-Gracia1 and Isabel Castrejon8, 1Hospital General Universitario Gregorio Marañón, Madrid, Spain, 2Instituto de Investigación Sanitaria Gregorio Marañón (IiSGM), Madrid, Spain, 3University Hospital Príncipe de Asturias, Immune System Diseases-Rheumatology Service, Alcalá de Henares, Madrid, Spain, 4Department of Rheumatology, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain, 5Department of Rheumatology, Hospital del Mar, Barcelona, Spain, 6Department of Rheumatology, Hospital de Basurto, Bilbao, Spain, 7Hospital General Universitario Alicante, Alicante, Spain, 8Hospital Universitario Gregorio Marañón, Madrid, Spain
Background/Purpose: To investigate the occurrence and relative risk of incident malignancy in patients with rheumatic diseases and previous malignancy treated with biologic and targeted synthetic DMARDs (b/tsDMARDs)
Methods: Cohort study of patients included in BIOBADASER 3.0 up to 2021, treated with b/tsDMARDs and history of previous malignancy. Incident cancer was defined as any cancer (new primaries, local recurrence or metastases) during the drug exposure leading to therapy discontinuation. Incidence rates ratios of cancer per 1,000 patients-year (PY) and 95% confidence interval (CI) were estimated. Rates of incident cancer in tsDMARDs and other bDMARDs versus TNFi were compared.
Results: A total of 352 patients over 9,129 patients from BIOBADASER 3.0 had history of previous malignancy. Overall, there were 32 incident malignancies (17 solid cancer, 14 non-melanoma skin cancer and 1 melanoma) (Figure 1). Baseline characteristics of patients are shown in Table 1. The overall rate of incident malignancy was 27.1 (95% CI 18.6-38.3) events/1,000 PY, ranging between none events/1000 PY in the anti-IL17 group to 51.7 events/1000 PY in the anti-CTLA-4 group (Table 2). The overall rate of incident cancer did not differ significantly in patients exposed to JAKi [0.6 (95% CI 0.1-2.5)], anti-CD20 [0.3 (95% CI 0.1-1.4)], anti-IL6 [1.2 (95% CI 0.5-3.4)] or anti-CTLA-4 [1.3 (95% CI 0.5-3.6) versus TNFi therapy. The rate of different types of cancer (melanoma, non-melanoma skin cancer or solid tumors) did not differ between the different treatment groups when compared to TNFi.
Conclusion: The risk of incident cancer in patients with rheumatic diseases and previous malignancy did not differ between TNFi or other b/tsDMARDs
.jpg) Table 1. Baseline characteristics of patients with prior malignancy with exposure to bDMARDs and tsDMARDs
Table 1. Baseline characteristics of patients with prior malignancy with exposure to bDMARDs and tsDMARDs
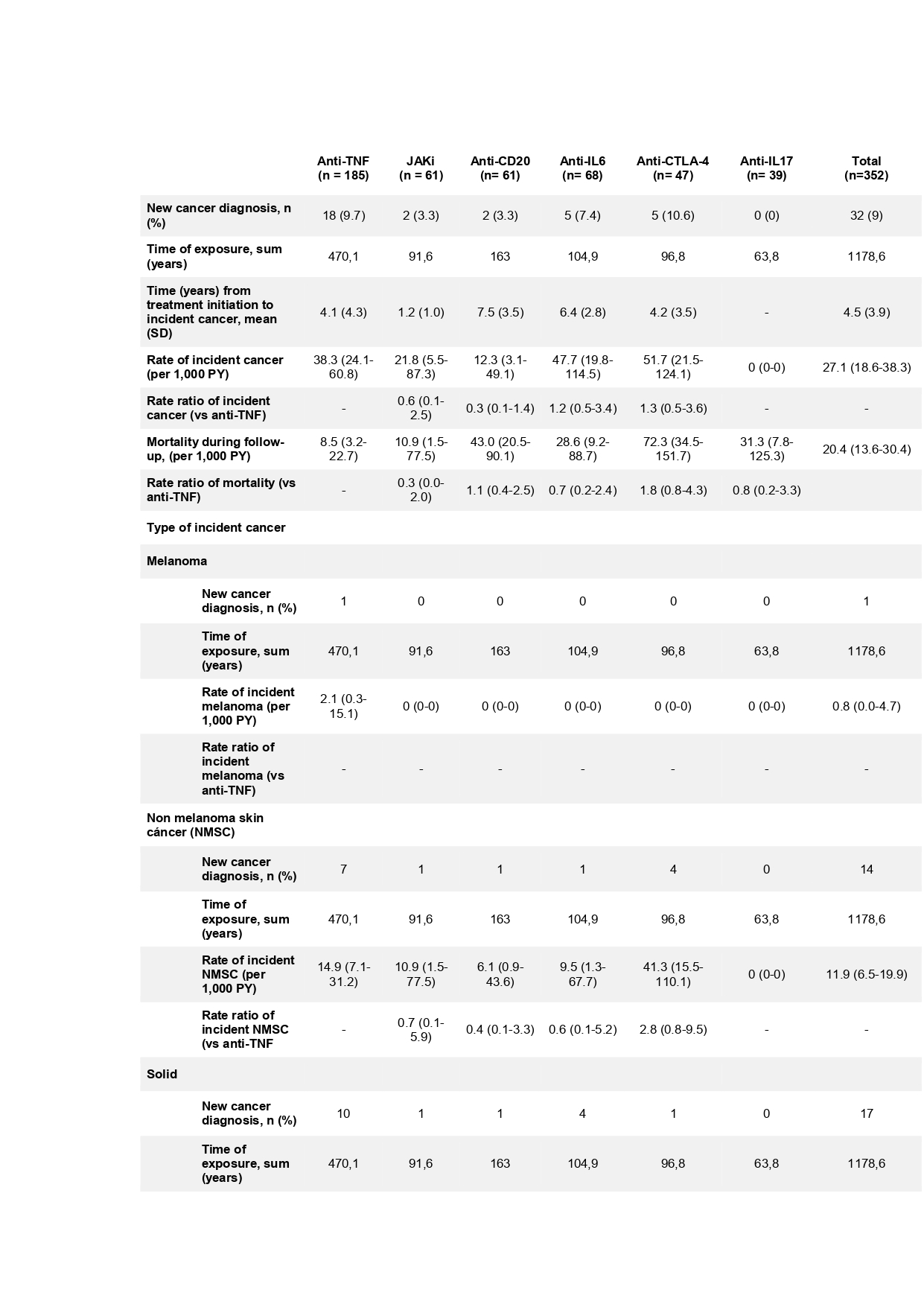 Table 2. Rate and type of incident cancer in patients with prior malignancy with exposure to bDMARDs and tsDMARDs
Table 2. Rate and type of incident cancer in patients with prior malignancy with exposure to bDMARDs and tsDMARDs
 Figure 1. Flowchart of patients included in the study.
Figure 1. Flowchart of patients included in the study.
Disclosures: J. Molina, None; F. Sanchez-Alonso, None; C. Bohorquez, None; C. Diaz-Torne, None; C. Perez-Garcia, None; J. Blanco-Madrigal, None; P. Vela-Casampere, None; J. Älvaro-Gracia, None; I. Castrejon, None.
Background/Purpose: To investigate the occurrence and relative risk of incident malignancy in patients with rheumatic diseases and previous malignancy treated with biologic and targeted synthetic DMARDs (b/tsDMARDs)
Methods: Cohort study of patients included in BIOBADASER 3.0 up to 2021, treated with b/tsDMARDs and history of previous malignancy. Incident cancer was defined as any cancer (new primaries, local recurrence or metastases) during the drug exposure leading to therapy discontinuation. Incidence rates ratios of cancer per 1,000 patients-year (PY) and 95% confidence interval (CI) were estimated. Rates of incident cancer in tsDMARDs and other bDMARDs versus TNFi were compared.
Results: A total of 352 patients over 9,129 patients from BIOBADASER 3.0 had history of previous malignancy. Overall, there were 32 incident malignancies (17 solid cancer, 14 non-melanoma skin cancer and 1 melanoma) (Figure 1). Baseline characteristics of patients are shown in Table 1. The overall rate of incident malignancy was 27.1 (95% CI 18.6-38.3) events/1,000 PY, ranging between none events/1000 PY in the anti-IL17 group to 51.7 events/1000 PY in the anti-CTLA-4 group (Table 2). The overall rate of incident cancer did not differ significantly in patients exposed to JAKi [0.6 (95% CI 0.1-2.5)], anti-CD20 [0.3 (95% CI 0.1-1.4)], anti-IL6 [1.2 (95% CI 0.5-3.4)] or anti-CTLA-4 [1.3 (95% CI 0.5-3.6) versus TNFi therapy. The rate of different types of cancer (melanoma, non-melanoma skin cancer or solid tumors) did not differ between the different treatment groups when compared to TNFi.
Conclusion: The risk of incident cancer in patients with rheumatic diseases and previous malignancy did not differ between TNFi or other b/tsDMARDs
.jpg) Table 1. Baseline characteristics of patients with prior malignancy with exposure to bDMARDs and tsDMARDs
Table 1. Baseline characteristics of patients with prior malignancy with exposure to bDMARDs and tsDMARDs Table 2. Rate and type of incident cancer in patients with prior malignancy with exposure to bDMARDs and tsDMARDs
Table 2. Rate and type of incident cancer in patients with prior malignancy with exposure to bDMARDs and tsDMARDs Figure 1. Flowchart of patients included in the study.
Figure 1. Flowchart of patients included in the study. Disclosures: J. Molina, None; F. Sanchez-Alonso, None; C. Bohorquez, None; C. Diaz-Torne, None; C. Perez-Garcia, None; J. Blanco-Madrigal, None; P. Vela-Casampere, None; J. Älvaro-Gracia, None; I. Castrejon, None.

