Back
Poster Session D
Session: (1830–1855) Miscellaneous Rheumatic and Inflammatory Diseases Poster III
1852: Clinical Efficacy and Safety of Guselkumab Maintenance Therapy in Patients with Moderately to Severely Active Crohn’s Disease: Week 48 Analyses from the Phase 2 GALAXI 1 Study
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- AS
Aparna Sahoo, DO
Janssen Pharmaceuticals
Springhouse, PA, United States
Abstract Poster Presenter(s)
Silvio Danese1, Remo Panaccione2, David T. Rubin3, Bruce E. Sands4, Walter Reinisch5, Geert D’Haens6, Julián Panés7, Susana Gonzalez8, Kathleen Weisel8, Aparna Sahoo8, Mary Ellen Frustaci8, Zijiang Yang8, William J. Sandborn9, Anita Afzali10, Tadakazu Hisamatsu11, Jane M. Andrews12 and Brian G. Feagan13, 1Gastroenterology and Endoscopy, IRCCS Ospedale San Raffaele and University Vita-Salute San Raffaele, Milano, Italy, 2Inflammatory Bowel Disease Group, University of Calgary, Calgary, AB, Canada, 3University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago, IL, 4Dr. Henry D. Janowitz Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, NY, USA, Chicago, IL, 5Division of Gastroenterology & Hepatology, Medical University of Vienna, Vienna, Austria, 6Department of Gastroenterology, Amsterdam University Medical Centers, Amsterdam, Netherlands, 7Hospital Clínic de Barcelona, IDIBAPS, CIBERehd, Barcelona, Spain, 8Immunology, Janssen Research & Development, LLC, Spring House, PA, 9Division of Gastroenterology, University of California San Diego, La Jolla, CA, USA, La Jolla, 10Wexner Medical Center, The Ohio State University, Columbus, OH, 11Department of Gastroenterology and Hepatology, Kyorin University, Tokyo, Japan, 12Department of Gastroenterology & Hepatology, Royal Adelaide Hospital & University of Adelaide, Adelaide, Australia, 13Western University and Senior Scientific Director, Alimentiv Inc (formerly Robarts Clinical Trials Inc), London, ON, Canada
Background/Purpose: GALAXI 1 is a Phase 2, double-blind, placebo (PBO)-controlled, multicenter study evaluating the efficacy/safety of guselkumab (GUS), a selective IL-23 p19 antagonist, in patients with moderately to severely active Crohn’s disease (CD) with inadequate response/intolerance to conventional therapies (corticosteroids, immunomodulators) and/or biologics (tumor necrosis factor antagonists, vedolizumab). At week (Wk) 12, all GUS induction doses (200, 600, and 1200 mg intravenous [IV]) had greater improvements vs PBO for key clinical/endoscopic outcomes. We report the clinical efficacy and safety of maintenance treatment through Wk 48.
Methods: GALAXI employed a treat-through design over 48 wks. In induction, patients were randomized to GUS 200, 600, or 1200 mg IV, ustekinumab (UST) ~6 mg/kg IV, or PBO IV. Patients transitioned to maintenance dosing as follows: PBO non-responders→UST ~6 mg/kg IV→90 mg subcutaneous (SC) every 8 weeks (q8w); PBO responders→PBO SC q4w, GUS 200 mg IV→100 mg SC q8w, GUS 600 mg IV→200 mg SC q4w, GUS 1200 mg IV→200 mg SC q4w, and UST ~6 mg/kg IV→90 mg SC q8w. Patients randomized to PBO were not included in the Wk 48 efficacy analyses. Primary and major secondary endpoints evaluated the efficacy of GUS vs PBO at Wk 12. Evaluations of Wk 48 endpoints were prespecified but not multiplicity controlled. UST was a reference arm; the study was not powered to evaluate differences between treatment groups with respect to efficacy at Wk 48.
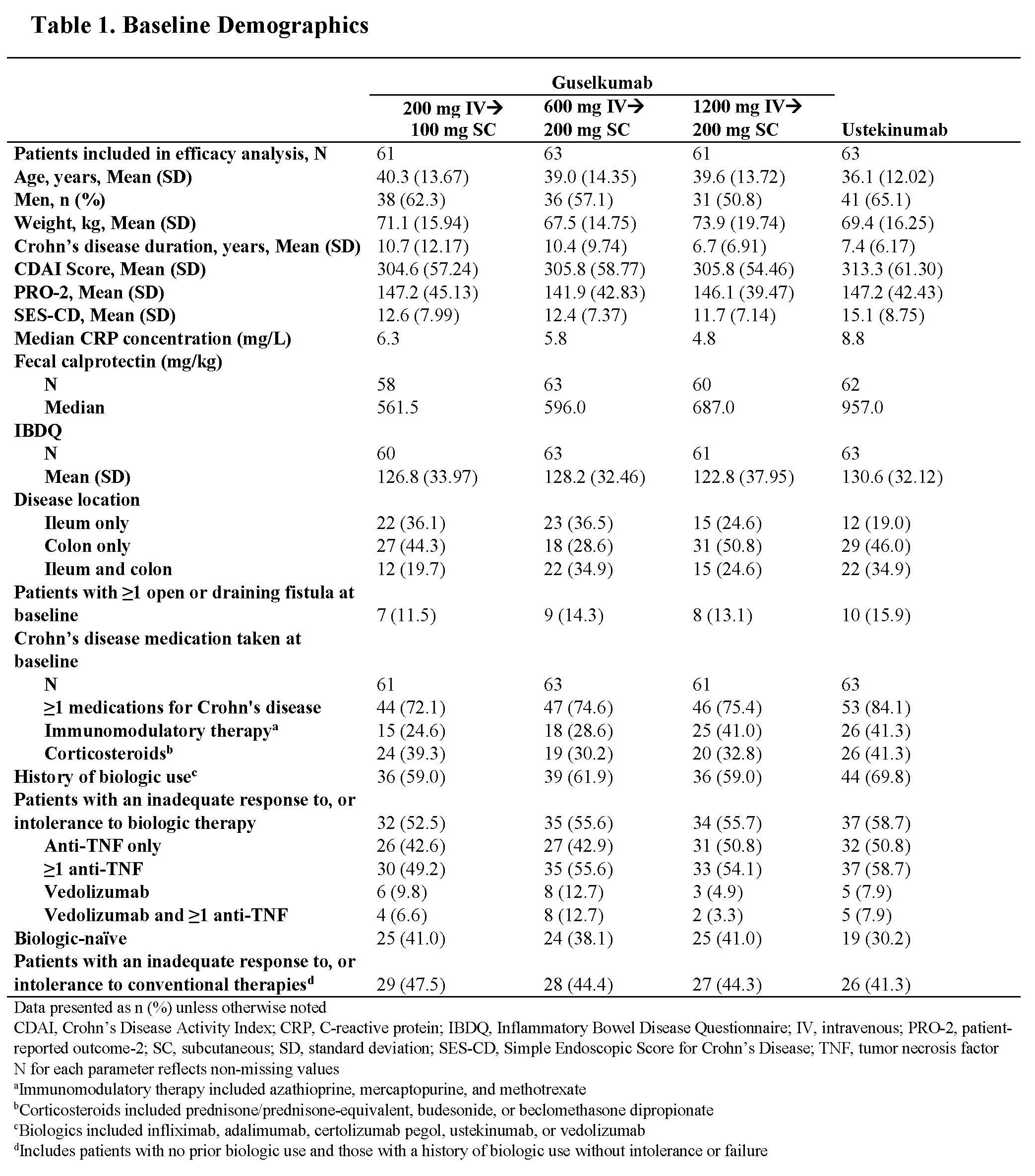
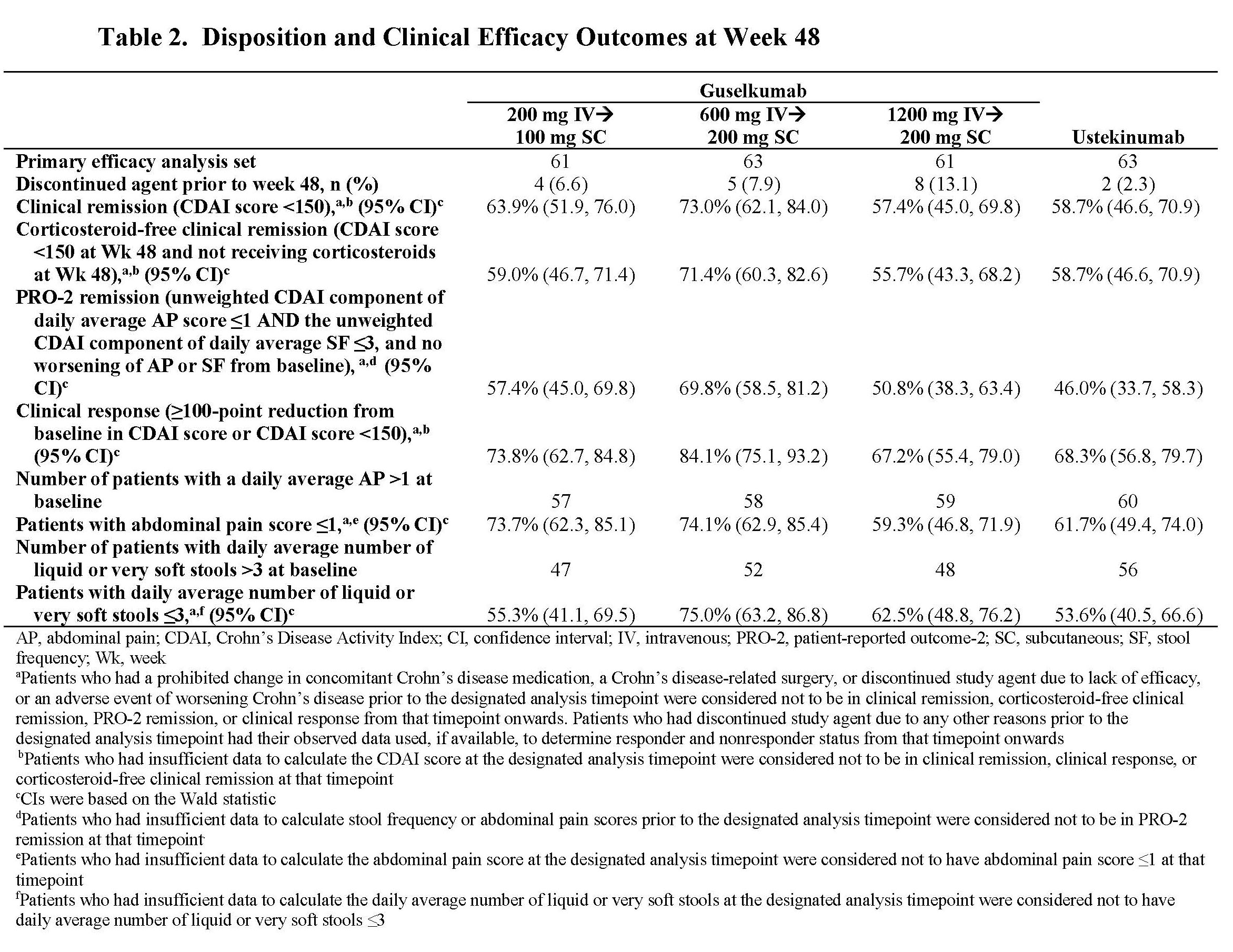
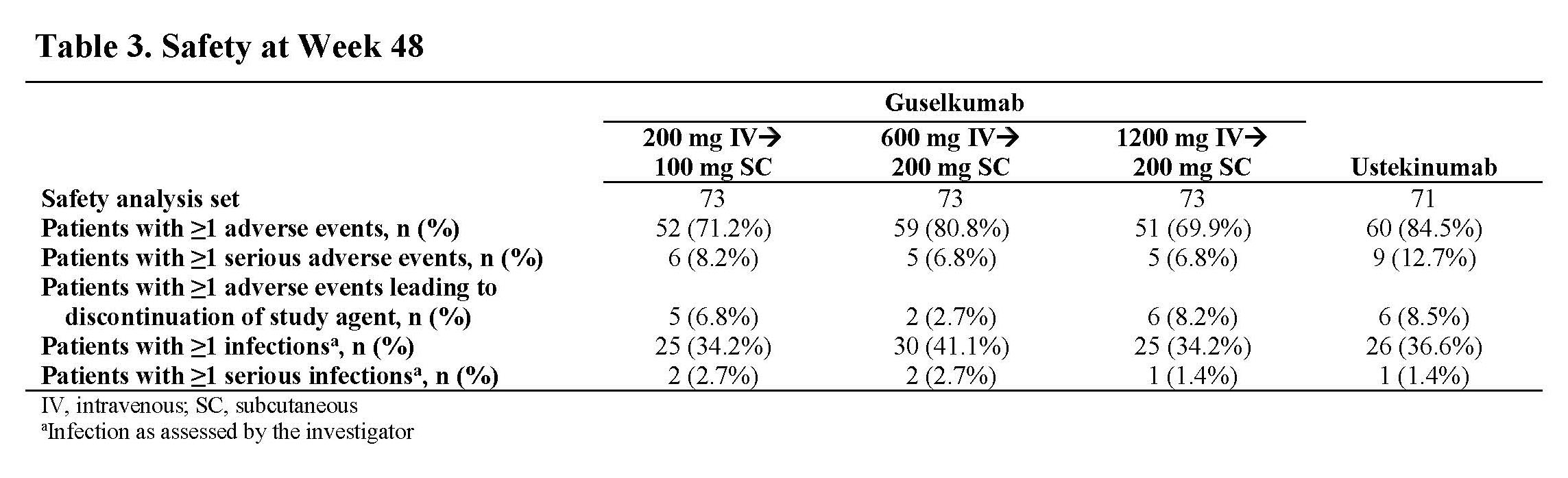
Results: Through Wk 48, 248 patients in the primary efficacy analysis set were randomized and evaluated. Baseline demographics were similar across groups (Table 1). Discontinuation rates were low across active treatment groups. No dose response was observed across clinical efficacy assessments (Table 2). Proportions of patients achieving clinical remission at Wk 48 ranged from 57.4% to 73.0% among GUS dose groups. The vast majority of patients in clinical remission were also in corticosteroid-free remission at Wk 48, with rates ranging from 55.7% to 71.4% among GUS dose groups. Patient-reported outcome (PRO)-2 remission rates ranged from 50.8% to 69.8%, and proportions of patients achieving clinical response ranged from 67.2% to 84.1% among GUS dose groups. Proportions of patients achieving abdominal pain scores ≤1 or daily average number of liquid or very soft stools ≤3 are presented in Table 2. Outcomes in the reference UST group are also shown in Table 2. Key safety event rates were similar among the GUS dose groups (Table 3); no opportunistic infections, cases of tuberculosis, or deaths were reported in any group.
Conclusion: In this treat-through Phase 2 study of patients with moderately to severely active CD, GUS was safe and effective. GUS induction followed by SC maintenance achieved high rates of clinical efficacy at Wk 48. Safety results were consistent with the known safety profile in approved indications.
Disclosures: S. Danese, Allergan, Amgen, AstraZeneca, Biogen, Boehringer-Ingelheim, Celgene, Celltrion Healthcare, Ferring, Gilead Sciences, Hospira, Inc., Janssen Research & Development, LLC, Johnson & Johnson Health Care Systems, Inc., Pfizer, Sandoz, UCB, Vifor International Inc.; R. Panaccione, AbbVie, Ferring, Janssen, Pfizer, Takeda, Arena, Celgene, Eli Lilly, Gilead Sciences, Merck, Roche, Sandoz, Shire, Amgen, Bristol-Myers Squibb(BMS), Celltrion, Galapagos, Genentech, GlaxoSmith Kline, Mylan, Oppilan Pharma, Pandion Pharma, Sublimity Therapeutics, Theravance, Abbott, Alimentiv (formerly Robarts), AstraZeneca, Boehringer-Ingelheim, Cosmos Pharmaceuticals, Eisai, Elan, Progenity, Protagonist Therapeutics, Satisfai Health, Schering-Plough, UCB; D. Rubin, Takeda, Cornerstones Health, Inc, AbbVie, Altrubio, Allergan, Inc., Arena Pharmaceuticals, Aslan Pharmaceuticals, Athos Therapeutics, Bellatrix Pharmaceuticals, Boehringer Ingelheim, Ltd., Bristol-Myers Squibb(BMS), Celgene Corp/Syneos, Connect Biopharma, GalenPharma/Atlantica, Genentech/Roche, InDex Pharmaceuticals, Ironwood Pharmaceuticals, Iterative Scopes, Janssen Pharmaceuticals, Eli Lilly, Materia Prima, Pfizer, Prometheus Biosciences, Reistone, Techlab, Inc; B. Sands, Takeda, Pfizer, Theravance Biopharma R&D, Janssen, Vivante Health, Ventyx Biosciences, 4D Pharma, Abivax, Abbvie, Alimentiv, Allergan, Amgen, Arena Pharmaceuticals, AstraZeneca, Bacain Therapeutics, Boehringer-Ingelheim, Boston Pharmaceuticals, Bristol-Myers Squibb(BMS), Calibr, Capella Bioscience, Celgene, Celltrion Healthcare, ClostraBio, Enthera, F.Hoffmann-La Roche, Ferring, Galapagos, Gilead, GlaxoSmithKline, GossamerBio, Immunic, Index Pharmaceuticals, Innovation Pharmaceuticals, Ironwood Pharmaceuticals, Kaleido, Kallyope, Lilly, MiroBio, Morphic Therapeutic, Oppilan Pharma, OSE Immunotherapeutics, Otsuka, Palantin Technologies, Progenity, Prometheus Biosciences, Protagonist Therapeutics, Q32 Bio, Prometheus Laboratories, Redhill Biopharma, Rheos Pharma, Salix Pharmaceuticals, Seres Therapeutics, Shire, Sienna Biopharmaceuticals, Sun Pharma, Surrozen, Target PharmaSolutions, Teva Branded Pharmaceutical Products R&D, Thelium, TLL Pharma, USWM Enterprises, Viela Bio, Vivelix Pharmaceuticals; W. Reinisch, Abbott Laboratories, AbbVie, Aesca, Centocor, Falk Pharma GmbH, Immundiagnostik, Janssen, Sandoz, Takeda, Aptalis, Astellas, Celltrion, Danone Austria, Elan, Ferring, Mitsubishi Tanabe Pharma Corporation, MSD, Otsuka, PDL, Pharmacosmos, PLS Education, Schering-Plough, Shire, Therakos, Vifor, Yakult, Amgen, AM Pharma, AstraZeneca, Biogen IDEC, Boehringer-Ingelheim, Bristol-Myers Squibb(BMS), Cellerix, Chemocentryx, Celgene, DSM, Galapagos, Genentech, Grünenthal, Inova, Johnson & Johnson, Kyowa Hakko Kirin Pharma, Lipid Therapeutics, Millenium, Nestlé, Novartis, Ocera, Pfizer, Procter & Gamble, Prometheus, Second Genome, Setpointmedical, Tigenix, UCB, Zealand, Zyngenia, 4SC, Algernon, AMT, AOP Orphan, Arena Pharmaceuticals, Avaxia, Roger Berger GmBH, Bioclinica, Covance, Eli Lilly, Ernest & Young, Gatehouse Bio Inc., Gilead, ICON, Index Pharma, Intrinsic Imaging, LivaNova, Mallinckrodt, Medahead, MedImmune, Nash Pharmaceuticals, Nippon Kayaku, OMass, Parexel, Periconsulting, Philip Morris Institute, Protagonist, Provention, Quell Therapeutics, Robarts Clinical Trial, Seres Therapeutics, Sigmoid, Sublimity, Theravance; G. D’Haens, Janssen; J. Panés, AbbVie, Pfizer, Janssen, Merck, Shire, Takeda, Theravance, Arena, Boehringer-Ingelheim, Celgene, Celltrion, Ferring, Genentech, GlaxoSmithKline, GoodGut, Nestlé, Origo, Pandion, Progenity, Robarts Clinical Trials, Roche, Wassermann; S. Gonzalez, Janssen Research & Development, LLC; K. Weisel, Janssen Research & Development, LLC; A. Sahoo, Janssen Research & Development, LLC; M. Frustaci, Janssen Research & Development, LLC; Z. Yang, Janssen Research & Development, LLC; W. Sandborn, AbbVie, Abivax, Arena Pharmaceuticals, Celgene, Genentech, Gilead Sciences, GlaxoSmithKlein(GSK), Janssen, Eli Lilly, Pfizer, Prometheus Biosciences, Seres Therapeutics, Shire, Takeda, Theravance Biopharma, Allakos, BeiGene, GossamerBio, Oppilan Pharma, Prometheus Laboratories, Progenity, Shoreline Biosciences, Ventyx Biosciences, Vimalan Biosciences, Vivreon Biosciences, Iveric Bio, Admirx, Alfasigma, Alimentiv (previously Robarts Clinical Trials, owned by Alimentiv Health Trust), Alivio Therapeutics, Amgen, Applied Molecular Transport, Bausch Health (Salix), Bellatrix Pharmaceuticals, Boehringer-Ingelheim, Boston Pharmaceuticals, Bristol-Myers Squibb(BMS), Celltrion, Cellularity, Cosmo Pharmaceuticals, Escalier Biosciences, Equillium, Forbion, Roche, Glenmark Pharmaceuticals, Immunic (Vital Therapies), Index Pharmaceuticals, Intact Therapeutics, Kyverna Therapeutics, Landos Biopharma, Otsuka, Pandion Therapeutics, Protagonist Therapeutics, Provention Bio, Reistone Biopharma, Shanghai Pharma Biotherapeutics, Sublimity Therapeutics, Surrozen, Thetis Pharmaceuticals, Tillotts Pharma, UCB, Vedanta Biosciences, Vivelix Pharmaceuticals, Zealand Pharma; A. Afzali, AbbVie, Takeda, Janssen, Bristol-Myers Squibb(BMS)/Celgene, Pfizer, Eli Lilly, Gilead, DiaSorin, TLL Pharmaceuticals, IBD Horizons, Bristol-Myers Squibb(BMS); T. Hisamatsu, Alfresa Pharma Co. Ltd., EA Pharma Co. Ltd., Mitsubishi Tanabe Pharma Corporation, AbbVie GK, Zeria Pharmaceutical Co. Ltd., Daiichi-Sankyo, Kyorin Pharmaceutical Co. Ltd., Nippon Kayaku Co. Ltd., Takeda Pharmaceutical Co. Ltd., Pfizer Inc., Mochida Pharmaceutical Co. Ltd., JIMRO Co. Ltd., Celgene K.K., Janssen Pharmaceutical K.K., Nichi-Iko Pharmaceutical Co. Ltd.; J. Andrews, Abbott, AbbVie, Allergan, Anatara, AstraZeneca, Bayer, BMS, Celegene, Celltrion, Falk, Ferring, Gilead, Hospira, Immunic, Janssen, MSD, Nestlé, Pfizer, Sandoz, Shire, Takeda, Vifor, Novartis; B. Feagan, Robarts Clinical Trials Inc., Allergan, Amgen, AstraZeneca/MedImmune Ltd., Atlantic Pharma, Avaxia Biologics Inc., Boehringer-Ingelheim, Bristol-Myers Squibb(BMS), Celgene, Centocor Inc., Galapagos, Johnson & Johnson/Janssen, Nestlé, Novartis, Abbott/AbbVie, Pfizer, Prometheus Laboratories, Protagonist, Salix Pharma, Sterna Biologicals, Takeda, Teva, TiGenix, UCB Pharma, Tillotts, AbbVie Inc., Amgen Inc., Atlantic Pharmaceuticals Ltd., Celgene Corporation, Celltech, Genentech Inc/Hoffman-La Roche Ltd, Gilead Sciences Inc., GlaxoSmithKline, Janssen Research & Development, LLC, Pfizer Inc, Receptos Inc./Celgene International, Sanofi, Santarus Inc., Takeda Development Center Americas Inc., Tillotts Pharma AG, UCB, AdMIRx Inc., Akebia Therapeutics, Applied Molecular Transport Inc., Aptevo Therapeutics, Asta Pharma, Avir Pharma, Biogen Idec, BioMx Israel, Boston Pharmaceuticals, Calypso Biotech, EnGene, Ferring Pharma, Galen/Atlantica, GiCare Pharma, Gilead, Gossamer Pharma, Inception IBD Inc, Intact Therapeutics, Kyowa Kakko Kirin Co Ltd., Lexicon, Eli Lilly, Lycera BioTech, Merck, Mesoblast Pharma, Millennium, Nextbiotix, Novonordisk, ParImmune, Parvus Therapeutics Inc., Prometheus Therapeutics and Diagnostics, Progenity, Qu Biologics, Rebiotix, Receptos, Shire, Sienna Biologics, Sigmoid Pharma, Synergy Pharma Inc., Teva Pharma, Vertex Pharma, Vivelix Pharma, VHsquared Ltd., Zyngenia, AstraZeneca, Elan/Biogen, Genentech/Roche.
Background/Purpose: GALAXI 1 is a Phase 2, double-blind, placebo (PBO)-controlled, multicenter study evaluating the efficacy/safety of guselkumab (GUS), a selective IL-23 p19 antagonist, in patients with moderately to severely active Crohn’s disease (CD) with inadequate response/intolerance to conventional therapies (corticosteroids, immunomodulators) and/or biologics (tumor necrosis factor antagonists, vedolizumab). At week (Wk) 12, all GUS induction doses (200, 600, and 1200 mg intravenous [IV]) had greater improvements vs PBO for key clinical/endoscopic outcomes. We report the clinical efficacy and safety of maintenance treatment through Wk 48.
Methods: GALAXI employed a treat-through design over 48 wks. In induction, patients were randomized to GUS 200, 600, or 1200 mg IV, ustekinumab (UST) ~6 mg/kg IV, or PBO IV. Patients transitioned to maintenance dosing as follows: PBO non-responders→UST ~6 mg/kg IV→90 mg subcutaneous (SC) every 8 weeks (q8w); PBO responders→PBO SC q4w, GUS 200 mg IV→100 mg SC q8w, GUS 600 mg IV→200 mg SC q4w, GUS 1200 mg IV→200 mg SC q4w, and UST ~6 mg/kg IV→90 mg SC q8w. Patients randomized to PBO were not included in the Wk 48 efficacy analyses. Primary and major secondary endpoints evaluated the efficacy of GUS vs PBO at Wk 12. Evaluations of Wk 48 endpoints were prespecified but not multiplicity controlled. UST was a reference arm; the study was not powered to evaluate differences between treatment groups with respect to efficacy at Wk 48.
Results: Through Wk 48, 248 patients in the primary efficacy analysis set were randomized and evaluated. Baseline demographics were similar across groups (Table 1). Discontinuation rates were low across active treatment groups. No dose response was observed across clinical efficacy assessments (Table 2). Proportions of patients achieving clinical remission at Wk 48 ranged from 57.4% to 73.0% among GUS dose groups. The vast majority of patients in clinical remission were also in corticosteroid-free remission at Wk 48, with rates ranging from 55.7% to 71.4% among GUS dose groups. Patient-reported outcome (PRO)-2 remission rates ranged from 50.8% to 69.8%, and proportions of patients achieving clinical response ranged from 67.2% to 84.1% among GUS dose groups. Proportions of patients achieving abdominal pain scores ≤1 or daily average number of liquid or very soft stools ≤3 are presented in Table 2. Outcomes in the reference UST group are also shown in Table 2. Key safety event rates were similar among the GUS dose groups (Table 3); no opportunistic infections, cases of tuberculosis, or deaths were reported in any group.
Conclusion: In this treat-through Phase 2 study of patients with moderately to severely active CD, GUS was safe and effective. GUS induction followed by SC maintenance achieved high rates of clinical efficacy at Wk 48. Safety results were consistent with the known safety profile in approved indications.



Disclosures: S. Danese, Allergan, Amgen, AstraZeneca, Biogen, Boehringer-Ingelheim, Celgene, Celltrion Healthcare, Ferring, Gilead Sciences, Hospira, Inc., Janssen Research & Development, LLC, Johnson & Johnson Health Care Systems, Inc., Pfizer, Sandoz, UCB, Vifor International Inc.; R. Panaccione, AbbVie, Ferring, Janssen, Pfizer, Takeda, Arena, Celgene, Eli Lilly, Gilead Sciences, Merck, Roche, Sandoz, Shire, Amgen, Bristol-Myers Squibb(BMS), Celltrion, Galapagos, Genentech, GlaxoSmith Kline, Mylan, Oppilan Pharma, Pandion Pharma, Sublimity Therapeutics, Theravance, Abbott, Alimentiv (formerly Robarts), AstraZeneca, Boehringer-Ingelheim, Cosmos Pharmaceuticals, Eisai, Elan, Progenity, Protagonist Therapeutics, Satisfai Health, Schering-Plough, UCB; D. Rubin, Takeda, Cornerstones Health, Inc, AbbVie, Altrubio, Allergan, Inc., Arena Pharmaceuticals, Aslan Pharmaceuticals, Athos Therapeutics, Bellatrix Pharmaceuticals, Boehringer Ingelheim, Ltd., Bristol-Myers Squibb(BMS), Celgene Corp/Syneos, Connect Biopharma, GalenPharma/Atlantica, Genentech/Roche, InDex Pharmaceuticals, Ironwood Pharmaceuticals, Iterative Scopes, Janssen Pharmaceuticals, Eli Lilly, Materia Prima, Pfizer, Prometheus Biosciences, Reistone, Techlab, Inc; B. Sands, Takeda, Pfizer, Theravance Biopharma R&D, Janssen, Vivante Health, Ventyx Biosciences, 4D Pharma, Abivax, Abbvie, Alimentiv, Allergan, Amgen, Arena Pharmaceuticals, AstraZeneca, Bacain Therapeutics, Boehringer-Ingelheim, Boston Pharmaceuticals, Bristol-Myers Squibb(BMS), Calibr, Capella Bioscience, Celgene, Celltrion Healthcare, ClostraBio, Enthera, F.Hoffmann-La Roche, Ferring, Galapagos, Gilead, GlaxoSmithKline, GossamerBio, Immunic, Index Pharmaceuticals, Innovation Pharmaceuticals, Ironwood Pharmaceuticals, Kaleido, Kallyope, Lilly, MiroBio, Morphic Therapeutic, Oppilan Pharma, OSE Immunotherapeutics, Otsuka, Palantin Technologies, Progenity, Prometheus Biosciences, Protagonist Therapeutics, Q32 Bio, Prometheus Laboratories, Redhill Biopharma, Rheos Pharma, Salix Pharmaceuticals, Seres Therapeutics, Shire, Sienna Biopharmaceuticals, Sun Pharma, Surrozen, Target PharmaSolutions, Teva Branded Pharmaceutical Products R&D, Thelium, TLL Pharma, USWM Enterprises, Viela Bio, Vivelix Pharmaceuticals; W. Reinisch, Abbott Laboratories, AbbVie, Aesca, Centocor, Falk Pharma GmbH, Immundiagnostik, Janssen, Sandoz, Takeda, Aptalis, Astellas, Celltrion, Danone Austria, Elan, Ferring, Mitsubishi Tanabe Pharma Corporation, MSD, Otsuka, PDL, Pharmacosmos, PLS Education, Schering-Plough, Shire, Therakos, Vifor, Yakult, Amgen, AM Pharma, AstraZeneca, Biogen IDEC, Boehringer-Ingelheim, Bristol-Myers Squibb(BMS), Cellerix, Chemocentryx, Celgene, DSM, Galapagos, Genentech, Grünenthal, Inova, Johnson & Johnson, Kyowa Hakko Kirin Pharma, Lipid Therapeutics, Millenium, Nestlé, Novartis, Ocera, Pfizer, Procter & Gamble, Prometheus, Second Genome, Setpointmedical, Tigenix, UCB, Zealand, Zyngenia, 4SC, Algernon, AMT, AOP Orphan, Arena Pharmaceuticals, Avaxia, Roger Berger GmBH, Bioclinica, Covance, Eli Lilly, Ernest & Young, Gatehouse Bio Inc., Gilead, ICON, Index Pharma, Intrinsic Imaging, LivaNova, Mallinckrodt, Medahead, MedImmune, Nash Pharmaceuticals, Nippon Kayaku, OMass, Parexel, Periconsulting, Philip Morris Institute, Protagonist, Provention, Quell Therapeutics, Robarts Clinical Trial, Seres Therapeutics, Sigmoid, Sublimity, Theravance; G. D’Haens, Janssen; J. Panés, AbbVie, Pfizer, Janssen, Merck, Shire, Takeda, Theravance, Arena, Boehringer-Ingelheim, Celgene, Celltrion, Ferring, Genentech, GlaxoSmithKline, GoodGut, Nestlé, Origo, Pandion, Progenity, Robarts Clinical Trials, Roche, Wassermann; S. Gonzalez, Janssen Research & Development, LLC; K. Weisel, Janssen Research & Development, LLC; A. Sahoo, Janssen Research & Development, LLC; M. Frustaci, Janssen Research & Development, LLC; Z. Yang, Janssen Research & Development, LLC; W. Sandborn, AbbVie, Abivax, Arena Pharmaceuticals, Celgene, Genentech, Gilead Sciences, GlaxoSmithKlein(GSK), Janssen, Eli Lilly, Pfizer, Prometheus Biosciences, Seres Therapeutics, Shire, Takeda, Theravance Biopharma, Allakos, BeiGene, GossamerBio, Oppilan Pharma, Prometheus Laboratories, Progenity, Shoreline Biosciences, Ventyx Biosciences, Vimalan Biosciences, Vivreon Biosciences, Iveric Bio, Admirx, Alfasigma, Alimentiv (previously Robarts Clinical Trials, owned by Alimentiv Health Trust), Alivio Therapeutics, Amgen, Applied Molecular Transport, Bausch Health (Salix), Bellatrix Pharmaceuticals, Boehringer-Ingelheim, Boston Pharmaceuticals, Bristol-Myers Squibb(BMS), Celltrion, Cellularity, Cosmo Pharmaceuticals, Escalier Biosciences, Equillium, Forbion, Roche, Glenmark Pharmaceuticals, Immunic (Vital Therapies), Index Pharmaceuticals, Intact Therapeutics, Kyverna Therapeutics, Landos Biopharma, Otsuka, Pandion Therapeutics, Protagonist Therapeutics, Provention Bio, Reistone Biopharma, Shanghai Pharma Biotherapeutics, Sublimity Therapeutics, Surrozen, Thetis Pharmaceuticals, Tillotts Pharma, UCB, Vedanta Biosciences, Vivelix Pharmaceuticals, Zealand Pharma; A. Afzali, AbbVie, Takeda, Janssen, Bristol-Myers Squibb(BMS)/Celgene, Pfizer, Eli Lilly, Gilead, DiaSorin, TLL Pharmaceuticals, IBD Horizons, Bristol-Myers Squibb(BMS); T. Hisamatsu, Alfresa Pharma Co. Ltd., EA Pharma Co. Ltd., Mitsubishi Tanabe Pharma Corporation, AbbVie GK, Zeria Pharmaceutical Co. Ltd., Daiichi-Sankyo, Kyorin Pharmaceutical Co. Ltd., Nippon Kayaku Co. Ltd., Takeda Pharmaceutical Co. Ltd., Pfizer Inc., Mochida Pharmaceutical Co. Ltd., JIMRO Co. Ltd., Celgene K.K., Janssen Pharmaceutical K.K., Nichi-Iko Pharmaceutical Co. Ltd.; J. Andrews, Abbott, AbbVie, Allergan, Anatara, AstraZeneca, Bayer, BMS, Celegene, Celltrion, Falk, Ferring, Gilead, Hospira, Immunic, Janssen, MSD, Nestlé, Pfizer, Sandoz, Shire, Takeda, Vifor, Novartis; B. Feagan, Robarts Clinical Trials Inc., Allergan, Amgen, AstraZeneca/MedImmune Ltd., Atlantic Pharma, Avaxia Biologics Inc., Boehringer-Ingelheim, Bristol-Myers Squibb(BMS), Celgene, Centocor Inc., Galapagos, Johnson & Johnson/Janssen, Nestlé, Novartis, Abbott/AbbVie, Pfizer, Prometheus Laboratories, Protagonist, Salix Pharma, Sterna Biologicals, Takeda, Teva, TiGenix, UCB Pharma, Tillotts, AbbVie Inc., Amgen Inc., Atlantic Pharmaceuticals Ltd., Celgene Corporation, Celltech, Genentech Inc/Hoffman-La Roche Ltd, Gilead Sciences Inc., GlaxoSmithKline, Janssen Research & Development, LLC, Pfizer Inc, Receptos Inc./Celgene International, Sanofi, Santarus Inc., Takeda Development Center Americas Inc., Tillotts Pharma AG, UCB, AdMIRx Inc., Akebia Therapeutics, Applied Molecular Transport Inc., Aptevo Therapeutics, Asta Pharma, Avir Pharma, Biogen Idec, BioMx Israel, Boston Pharmaceuticals, Calypso Biotech, EnGene, Ferring Pharma, Galen/Atlantica, GiCare Pharma, Gilead, Gossamer Pharma, Inception IBD Inc, Intact Therapeutics, Kyowa Kakko Kirin Co Ltd., Lexicon, Eli Lilly, Lycera BioTech, Merck, Mesoblast Pharma, Millennium, Nextbiotix, Novonordisk, ParImmune, Parvus Therapeutics Inc., Prometheus Therapeutics and Diagnostics, Progenity, Qu Biologics, Rebiotix, Receptos, Shire, Sienna Biologics, Sigmoid Pharma, Synergy Pharma Inc., Teva Pharma, Vertex Pharma, Vivelix Pharma, VHsquared Ltd., Zyngenia, AstraZeneca, Elan/Biogen, Genentech/Roche.

