Back
Poster Session D
Sjögren's syndrome
Session: (2017–2051) Sjögren's Syndrome – Basic and Clinical Science Poster
2047: Discriminative Power of Salivary Gland Ultrasound in Relation to Symptom-based Endotypes in Suspected and Definite Primary Sjögren’s Syndrome
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- LD
Liselotte Deroo, MD
Ghent University
Gent, Belgium
Abstract Poster Presenter(s)
Liselotte Deroo1, Helena Achten2, Eva Genbrugge2, Wouter Bauters2, Dimitri Roels2, Frederick Dochy2, David Creytens2, Ann-Sophie Kathleen De Craemer2, Filip Van den bosch3, Dirk Elewaut4 and isabelle peene5, 1Ghent University, Gent, Belgium, 2Ghent University Hospital, Gent, Belgium, 3Department of Internal Medicine and Paediatrics, Ghent University and VIB Centre for Inflammation Research, Ghent, Belgium, 4Department of Rheumatology, Ghent University Hospital, Belgium, VIB-UGent Center for Inflammation Research, Ghent University, Heusden, Belgium, 5Ghent University Hospital, Ghent, Belgium
Background/Purpose: Primary Sjögren's Syndrome (pSS) is characterized by xerophthalmia, xerostomia and increased lymphoma risk. To address heterogeneity in symptoms, Tarn et al. applied cluster analysis on a pSS population. They identified four subgroups based on patient-reported outcomes assessing dryness, fatigue, pain, anxiety and depression: low symptom burden (LSB), pain dominant with fatigue (PDF), dryness dominant with fatigue (DDF) and high symptom burden (HSB). These groups were considered true endotypes due to differences in hematological, biological, autoimmune and transcriptomic parameters.
Salivary gland ultrasound (SGUS) is emerging as essential tool in the evaluation of pSS, but its link to these endotypes is unknown. Therefore, we explored SGUS outcomes in relation to endotypes in patients with definite and suspected pSS.
Methods: Definite pSS patients (n=171) fulfilling the 2016 ACR/EULAR classification criteria, and suspected pSS patients (n=119), positive for at least one criterion, were included in the Belgian Sjögren's Syndrome Transition Trial (BeSSTT). Both patient groups were stratified into endotypes using the Newcastle Sjögren's Stratification Tool. SGUS was assessed using Hocevar score (0-48); scores ≥17 were considered positive. Categories were created with 0-14 indicating negative, 15-26 low positive and 27-48 high positive results. The dataset was randomly divided into a discovery (n=203) and replication (n=87) cohort.
Results: In the discovery cohort, Hocevar was significantly higher amongst definite than suspected pSS patients (21 (11-36) vs 10 (2-18); p< 0.001). This was also true when comparing definite and suspected pSS subgroups within each endotype, with the exception of LSB (17 (5-31) vs 19 (4-28); p=0.823) (Figure 1). SGUS had strong discriminative power for pSS classification (AUC=0.74), especially in DDF (AUC=0.89). In definite pSS, Hocevar scores were strikingly high in DDF compared to other endotypes (38 (20-44) vs 18 (9-33); p< 0.001). Additionally, a significantly higher proportion of DDF patients had a high positive Hocevar score compared to other patients (66.7% vs 28.0%; p< 0.001) (Figure 2). Patients with high positive SGUS-scores reported a significantly higher dryness burden (p< 0.001), and had lower unstimulated salivary flow rates (p=0.005) and higher ocular staining scores (p< 0.001) than those with a negative SGUS. They had significantly lower lymphocyte counts (p=0.005), while β2-microglobuline, IgG and rheumatoid factor levels were higher (all p< 0.001), independent of endotype. Moreover, a subset of young, anti-SSA/Ro positive patients not fulfilling classification criteria showed clear SGUS abnormalities. Replication showed similar results.
Conclusion: Amongst definite pSS patients, SGUS-scores were highest in DDF compared to other endotypes, providing the first evidence of imaging abnormalities in salivary glands matching distinct biological profiles ascribed to pSS endotypes. Additionally, a subset of patients with potential early disease was detected due to presence of anti-SSA antibodies and high SGUS-scores. These results underscore the role of SGUS as powerful tool both in pSS classification and stratification.
.jpg) Figure 1 Hocevar scores for different endotypes in definite and suspected pSS patients in the discovery (A) and replication (B) cohort. Within the definite pSS subgroup, differences between each endotype and patients from the other endotypes were assessed by Man-Whitney U testing. Significant differences were indicated with the corresponding p-value.
Figure 1 Hocevar scores for different endotypes in definite and suspected pSS patients in the discovery (A) and replication (B) cohort. Within the definite pSS subgroup, differences between each endotype and patients from the other endotypes were assessed by Man-Whitney U testing. Significant differences were indicated with the corresponding p-value.
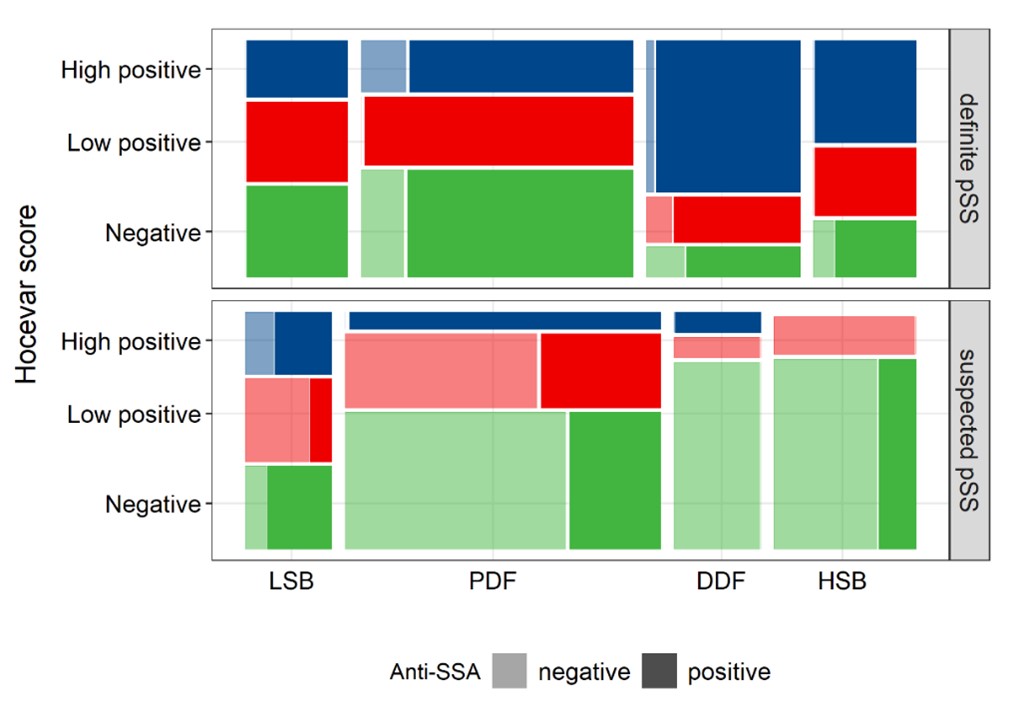 Figure 2 Mosaic plot visualizing the proportion of negative (0-14), low positive (15-26) and high positive (27-48) Hocevar scores for all endotypes both in definite and suspected pSS in the discovery cohort. Darker shades indicate the proportion anti-SSA/Ro positive patients in each subgroup. The area of each rectangle represents the number of patients in each subgroup. Abbreviations: LSB=low symptom burden; PDF=pain dominant with fatigue; DDF=dry dominant with fatigue; HSB=high symptom burden.
Figure 2 Mosaic plot visualizing the proportion of negative (0-14), low positive (15-26) and high positive (27-48) Hocevar scores for all endotypes both in definite and suspected pSS in the discovery cohort. Darker shades indicate the proportion anti-SSA/Ro positive patients in each subgroup. The area of each rectangle represents the number of patients in each subgroup. Abbreviations: LSB=low symptom burden; PDF=pain dominant with fatigue; DDF=dry dominant with fatigue; HSB=high symptom burden.
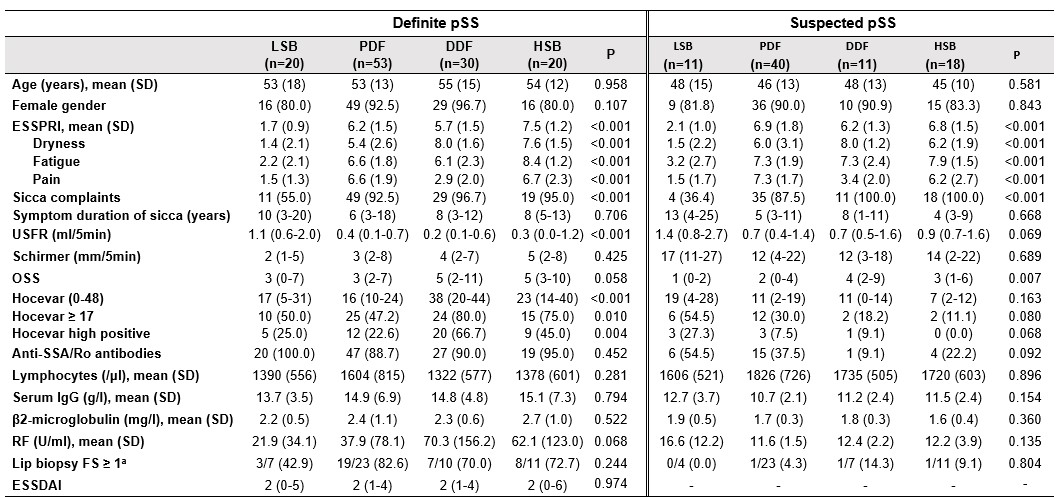 Table 1 Clinical characteristics and laboratory parameters of the discovery cohort. Continuous data presented as median (IQR) and categorical data as number of patients (%) unless otherwise indicated. P-values refer to comparison of endotypes within definite and suspected pSS subgroups, based on Kruskal-Wallis or Chi-squared tests. aResults are reported as the ratio aberrant on available biopsies. Abbreviations: ESSPRI=EULAR Sjögren's syndrome patient reported index; USFR=unstimulated salivary flow rate; OSS=ocular staining score; FS=focus score; ESSDAI=EULAR Sjögren's syndrome disease activity index.
Table 1 Clinical characteristics and laboratory parameters of the discovery cohort. Continuous data presented as median (IQR) and categorical data as number of patients (%) unless otherwise indicated. P-values refer to comparison of endotypes within definite and suspected pSS subgroups, based on Kruskal-Wallis or Chi-squared tests. aResults are reported as the ratio aberrant on available biopsies. Abbreviations: ESSPRI=EULAR Sjögren's syndrome patient reported index; USFR=unstimulated salivary flow rate; OSS=ocular staining score; FS=focus score; ESSDAI=EULAR Sjögren's syndrome disease activity index.
Disclosures: L. Deroo, None; H. Achten, None; E. Genbrugge, None; W. Bauters, None; D. Roels, None; F. Dochy, None; D. Creytens, None; A. De Craemer, None; F. Van den bosch, AbbVie, Lilly, Galapagos, Janssen, Merck, Novartis, Pfizer, UCB, Amgen, Bristol-Myers Squibb(BMS), Celgene; D. Elewaut, AbbVie, Eli Lilly, Galapagos, Novartis, UCB Pharma; i. peene, None.
Background/Purpose: Primary Sjögren's Syndrome (pSS) is characterized by xerophthalmia, xerostomia and increased lymphoma risk. To address heterogeneity in symptoms, Tarn et al. applied cluster analysis on a pSS population. They identified four subgroups based on patient-reported outcomes assessing dryness, fatigue, pain, anxiety and depression: low symptom burden (LSB), pain dominant with fatigue (PDF), dryness dominant with fatigue (DDF) and high symptom burden (HSB). These groups were considered true endotypes due to differences in hematological, biological, autoimmune and transcriptomic parameters.
Salivary gland ultrasound (SGUS) is emerging as essential tool in the evaluation of pSS, but its link to these endotypes is unknown. Therefore, we explored SGUS outcomes in relation to endotypes in patients with definite and suspected pSS.
Methods: Definite pSS patients (n=171) fulfilling the 2016 ACR/EULAR classification criteria, and suspected pSS patients (n=119), positive for at least one criterion, were included in the Belgian Sjögren's Syndrome Transition Trial (BeSSTT). Both patient groups were stratified into endotypes using the Newcastle Sjögren's Stratification Tool. SGUS was assessed using Hocevar score (0-48); scores ≥17 were considered positive. Categories were created with 0-14 indicating negative, 15-26 low positive and 27-48 high positive results. The dataset was randomly divided into a discovery (n=203) and replication (n=87) cohort.
Results: In the discovery cohort, Hocevar was significantly higher amongst definite than suspected pSS patients (21 (11-36) vs 10 (2-18); p< 0.001). This was also true when comparing definite and suspected pSS subgroups within each endotype, with the exception of LSB (17 (5-31) vs 19 (4-28); p=0.823) (Figure 1). SGUS had strong discriminative power for pSS classification (AUC=0.74), especially in DDF (AUC=0.89). In definite pSS, Hocevar scores were strikingly high in DDF compared to other endotypes (38 (20-44) vs 18 (9-33); p< 0.001). Additionally, a significantly higher proportion of DDF patients had a high positive Hocevar score compared to other patients (66.7% vs 28.0%; p< 0.001) (Figure 2). Patients with high positive SGUS-scores reported a significantly higher dryness burden (p< 0.001), and had lower unstimulated salivary flow rates (p=0.005) and higher ocular staining scores (p< 0.001) than those with a negative SGUS. They had significantly lower lymphocyte counts (p=0.005), while β2-microglobuline, IgG and rheumatoid factor levels were higher (all p< 0.001), independent of endotype. Moreover, a subset of young, anti-SSA/Ro positive patients not fulfilling classification criteria showed clear SGUS abnormalities. Replication showed similar results.
Conclusion: Amongst definite pSS patients, SGUS-scores were highest in DDF compared to other endotypes, providing the first evidence of imaging abnormalities in salivary glands matching distinct biological profiles ascribed to pSS endotypes. Additionally, a subset of patients with potential early disease was detected due to presence of anti-SSA antibodies and high SGUS-scores. These results underscore the role of SGUS as powerful tool both in pSS classification and stratification.
.jpg) Figure 1 Hocevar scores for different endotypes in definite and suspected pSS patients in the discovery (A) and replication (B) cohort. Within the definite pSS subgroup, differences between each endotype and patients from the other endotypes were assessed by Man-Whitney U testing. Significant differences were indicated with the corresponding p-value.
Figure 1 Hocevar scores for different endotypes in definite and suspected pSS patients in the discovery (A) and replication (B) cohort. Within the definite pSS subgroup, differences between each endotype and patients from the other endotypes were assessed by Man-Whitney U testing. Significant differences were indicated with the corresponding p-value. Figure 2 Mosaic plot visualizing the proportion of negative (0-14), low positive (15-26) and high positive (27-48) Hocevar scores for all endotypes both in definite and suspected pSS in the discovery cohort. Darker shades indicate the proportion anti-SSA/Ro positive patients in each subgroup. The area of each rectangle represents the number of patients in each subgroup. Abbreviations: LSB=low symptom burden; PDF=pain dominant with fatigue; DDF=dry dominant with fatigue; HSB=high symptom burden.
Figure 2 Mosaic plot visualizing the proportion of negative (0-14), low positive (15-26) and high positive (27-48) Hocevar scores for all endotypes both in definite and suspected pSS in the discovery cohort. Darker shades indicate the proportion anti-SSA/Ro positive patients in each subgroup. The area of each rectangle represents the number of patients in each subgroup. Abbreviations: LSB=low symptom burden; PDF=pain dominant with fatigue; DDF=dry dominant with fatigue; HSB=high symptom burden. Table 1 Clinical characteristics and laboratory parameters of the discovery cohort. Continuous data presented as median (IQR) and categorical data as number of patients (%) unless otherwise indicated. P-values refer to comparison of endotypes within definite and suspected pSS subgroups, based on Kruskal-Wallis or Chi-squared tests. aResults are reported as the ratio aberrant on available biopsies. Abbreviations: ESSPRI=EULAR Sjögren's syndrome patient reported index; USFR=unstimulated salivary flow rate; OSS=ocular staining score; FS=focus score; ESSDAI=EULAR Sjögren's syndrome disease activity index.
Table 1 Clinical characteristics and laboratory parameters of the discovery cohort. Continuous data presented as median (IQR) and categorical data as number of patients (%) unless otherwise indicated. P-values refer to comparison of endotypes within definite and suspected pSS subgroups, based on Kruskal-Wallis or Chi-squared tests. aResults are reported as the ratio aberrant on available biopsies. Abbreviations: ESSPRI=EULAR Sjögren's syndrome patient reported index; USFR=unstimulated salivary flow rate; OSS=ocular staining score; FS=focus score; ESSDAI=EULAR Sjögren's syndrome disease activity index.Disclosures: L. Deroo, None; H. Achten, None; E. Genbrugge, None; W. Bauters, None; D. Roels, None; F. Dochy, None; D. Creytens, None; A. De Craemer, None; F. Van den bosch, AbbVie, Lilly, Galapagos, Janssen, Merck, Novartis, Pfizer, UCB, Amgen, Bristol-Myers Squibb(BMS), Celgene; D. Elewaut, AbbVie, Eli Lilly, Galapagos, Novartis, UCB Pharma; i. peene, None.

