Back

Poster Session D - Tuesday Morning
Category: IBD
D0385 - Ustekinumab versus Tofacitinib as Second-Line Therapy for Ulcerative Colitis: A Retrospective, Observational Study
Tuesday, October 25, 2022
10:00 AM – 12:00 PM ET
Location: Crown Ballroom

Has Audio
- JG
John Galatowitsch, MD
University of Colorado School of Medicine
Aurora, CO
Presenting Author(s)
John Galatowitsch, MD1, John P. Haydek, MD1, Waseem Ahmed, MD2, Blair Fennimore, MD1, Debbie Cheng, MD1, Calen A. Steiner, MD, MS1, Benjamin Click, MD3, Mark E. Gerich, MD, MBA1, Alexis Oonk, FNP1, Frank I. Scott, MD3
1University of Colorado School of Medicine, Aurora, CO; 2University of Colorado School of Medicine, Denver, CO; 3University of Colorado Anschutz Medical Campus, Aurora, CO
Introduction: Treatment options for ulcerative colitis (UC) have expanded rapidly over the past two decades. However, optimal positioning of these agents, particularly after previous biologic failure, remains uncertain. We sought to assess the clinical effectiveness and medication persistence rates of ustekinumab (UST) compared to tofacitinib (TOF) in UC patients with prior biologic exposure.
Methods: Patients with UC followed at a tertiary ambulatory referral center who were ≥18 years of age with previous failure of anti-TNF therapies or vedolizumab who then started UST or TOF were eligible for inclusion. Partial Mayo Scores, laboratory data including inflammatory markers, concomitant steroid use, and disease specific data were collected at baseline. The primary outcome was the percentage of individuals in clinical remission at 12 months after medication initiation, defined as a Partial Mayo Score of ≤3. Steroid-free remission and medication persistence at 12 months were also assessed. Rates of serious adverse events were compared between therapies. Clinical remission was measured at follow-up visits during the 12 months after initiation, with the last observation carried forward, and compared via Fisher’s exact test. Medication persistence was assessed via Kaplan-Meier curves and log-rank tests, censoring at the last known visit up to 12 months after initiation.
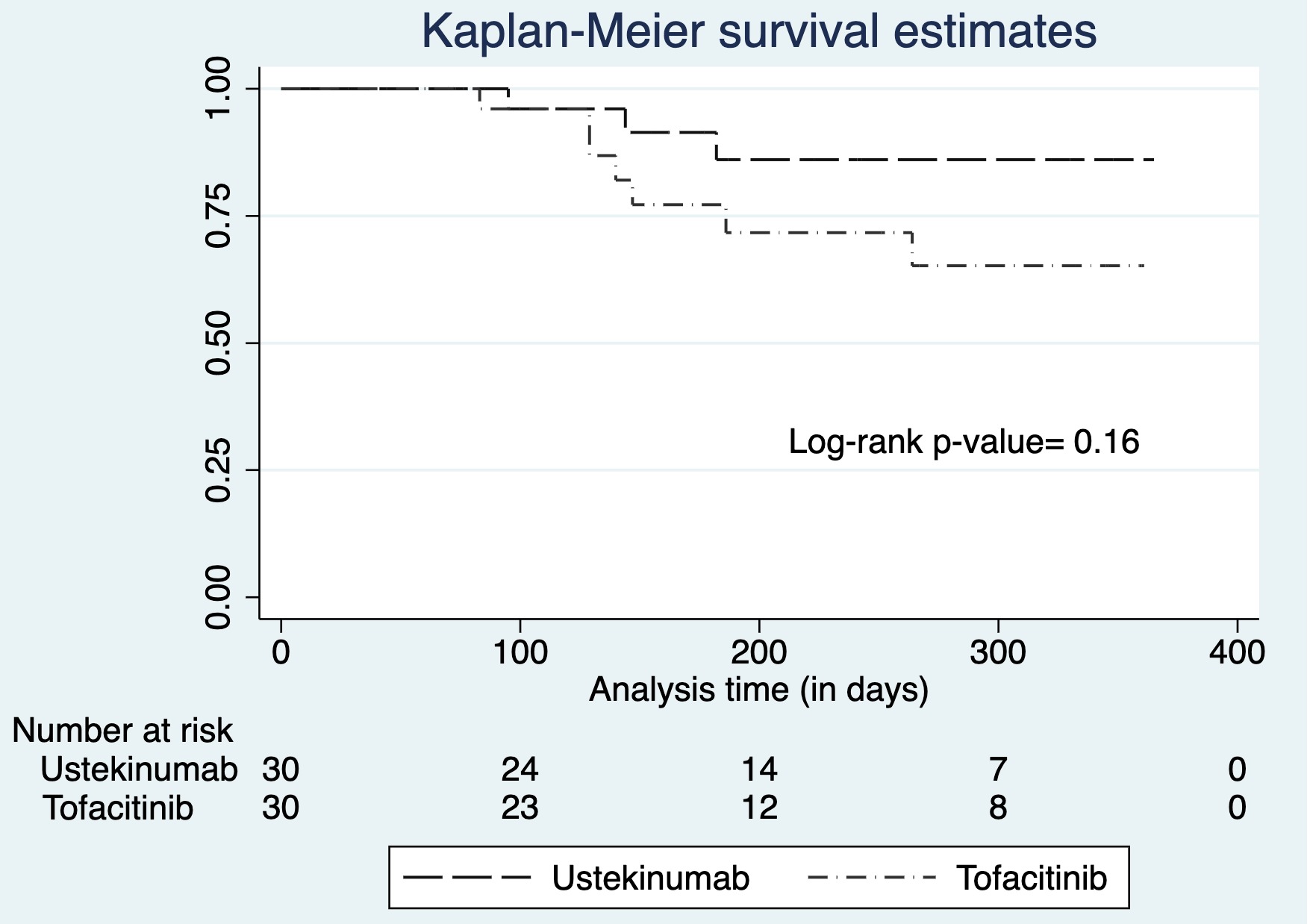
Results: Thirty patients initiating UST and 30 patients initiating TOF were identified from 2017 to 2021. Baseline demographics were similar between the UST and TOF treatment groups, though individuals receiving TOF had higher Partial Mayo Scores and a higher average number of prior biologics (Table 1). At 12 months, clinical remission rates were similar in those receiving UST compared to those initiating TOF (66.7% vs 56.7%; p=0.60), as were rates of steroid-free clinical remission (56.7% vs 43.3%; p= 0.44). Medication persistence rates were numerically higher with UST compared to TOF (90.0% vs 76.7%, p=0.16 (Figure 1). Adverse events were rare in both groups.
Discussion: In this retrospective cohort study, rates of clinical remission and medication persistence were similar between UST and TOF in individuals who had failed prior biologic therapy. Adverse event rates were similar between groups. Larger prospective studies with adjustment for confounding by channeling bias are required to best elucidate the ideal position for these therapies after failure of an initial biologic therapy.
Disclosures:
John Galatowitsch, MD1, John P. Haydek, MD1, Waseem Ahmed, MD2, Blair Fennimore, MD1, Debbie Cheng, MD1, Calen A. Steiner, MD, MS1, Benjamin Click, MD3, Mark E. Gerich, MD, MBA1, Alexis Oonk, FNP1, Frank I. Scott, MD3. D0385 - Ustekinumab versus Tofacitinib as Second-Line Therapy for Ulcerative Colitis: A Retrospective, Observational Study, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1University of Colorado School of Medicine, Aurora, CO; 2University of Colorado School of Medicine, Denver, CO; 3University of Colorado Anschutz Medical Campus, Aurora, CO
Introduction: Treatment options for ulcerative colitis (UC) have expanded rapidly over the past two decades. However, optimal positioning of these agents, particularly after previous biologic failure, remains uncertain. We sought to assess the clinical effectiveness and medication persistence rates of ustekinumab (UST) compared to tofacitinib (TOF) in UC patients with prior biologic exposure.
Methods: Patients with UC followed at a tertiary ambulatory referral center who were ≥18 years of age with previous failure of anti-TNF therapies or vedolizumab who then started UST or TOF were eligible for inclusion. Partial Mayo Scores, laboratory data including inflammatory markers, concomitant steroid use, and disease specific data were collected at baseline. The primary outcome was the percentage of individuals in clinical remission at 12 months after medication initiation, defined as a Partial Mayo Score of ≤3. Steroid-free remission and medication persistence at 12 months were also assessed. Rates of serious adverse events were compared between therapies. Clinical remission was measured at follow-up visits during the 12 months after initiation, with the last observation carried forward, and compared via Fisher’s exact test. Medication persistence was assessed via Kaplan-Meier curves and log-rank tests, censoring at the last known visit up to 12 months after initiation.
Results: Thirty patients initiating UST and 30 patients initiating TOF were identified from 2017 to 2021. Baseline demographics were similar between the UST and TOF treatment groups, though individuals receiving TOF had higher Partial Mayo Scores and a higher average number of prior biologics (Table 1). At 12 months, clinical remission rates were similar in those receiving UST compared to those initiating TOF (66.7% vs 56.7%; p=0.60), as were rates of steroid-free clinical remission (56.7% vs 43.3%; p= 0.44). Medication persistence rates were numerically higher with UST compared to TOF (90.0% vs 76.7%, p=0.16 (Figure 1). Adverse events were rare in both groups.
Discussion: In this retrospective cohort study, rates of clinical remission and medication persistence were similar between UST and TOF in individuals who had failed prior biologic therapy. Adverse event rates were similar between groups. Larger prospective studies with adjustment for confounding by channeling bias are required to best elucidate the ideal position for these therapies after failure of an initial biologic therapy.

Figure: Figure 1: Kaplan-Meier Survival Curves of medication persistence comparing ustekinumab to tofacitinib after prior biologic failure in ulcerative colitis
| Ustekinumab | Tofacitinib | |
| PATIENTS | 30 | 30 |
| AGE | ||
| Median, Years | 42.5 | 38.5 |
| IQR, Years | 32.5 - 55.0 | 29.0 - 46.5 |
| SEX | ||
| Male, Percent | 50.0 | 56.0 |
| Female, Percent | 50.0 | 44.0 |
| RACE | ||
| White, Percent | 86.7 | 93.3 |
| Asian, Percent | 3.3 | 0.0 |
| More Than One Race, Percent | 3.3 | 3.3 |
| Unknown Race, Percent | 6.7 | 3.3 |
| ETHNICITY | ||
| Hispanic or Latino, Percent | 10.0 | 6.7 |
| DISEASE DURATION | ||
| Median, Years | 6.0 | 6.0 |
| IQR, Years | 3.3 - 11.0 | 4.0 - 9.8 |
| DISEASE DISTRIBUTION | ||
| Extensive Colitis (E3), Percent | 63.3 | 83.3 |
| Left-Sided Colitis (E2), Percent | 36.7 | 16.7 |
| Proctosigmoiditis (E1), Percent | 0.0 | 0.0 |
| SMOKING STATUS | ||
| Active Smoker, Percent | 0.0 | 0.0 |
| PRIOR FAILED THERAPIES | ||
| Mean Number of Failed Biologic Therapies | 2.3 | 3.2 |
| Failed 1 Biologic, Percent | 53.3 | 20.0 |
| Failed 2 Biologics, Percent | 36.7 | 46.7 |
| Failed 3+ Biologics, Percent | 10.0 | 33.3 |
| Failed TNF Inhibitor, Percent | 76.7 | 96.7 |
| Failed Vedolizumab, Percent | 63.3 | 73.3 |
| Failed Immunosuppressant, Percent | 66.7 | 80.0 |
| BASELINE PARTIAL MAYO SCORE | ||
| Median | 4.0 | 5.5 |
| IQR | 3.0 - 6.0 | 4.0 - 7.0 |
| Baseline Rectal Bleeding, Percent | 43.8 | 73.3 |
| BASELINE INFLAMMATORY DATA | ||
| CRP, Mean | 11.1 | 12.9 |
| CRP, StDev | 16.0 | 14.5 |
| Fecal Calprotectin, Mean | 1546 | 1048 |
| Fecal Calprotectin, StDev | 1373 | 1121 |
| BASELINE STEROID USE | ||
| Steroid Utilization, Percent | 60.0 | 76.7 |
| Prednisone Utilization, Percent | 36.7 | 70.0 |
| Budesonide Utilization, Percent | 16.7 | 3.3 |
| BASELINE NON-BIOLOGIC THERAPIES | ||
| Immunosuppressant Utilization, Percent | 3.3 | 3.3 |
| Aminosalicylate Utilization, Percent | 10.0 | 10.0 |
Table: Table 1: Baseline Characteristics
Disclosures:
John Galatowitsch indicated no relevant financial relationships.
John Haydek indicated no relevant financial relationships.
Waseem Ahmed indicated no relevant financial relationships.
Blair Fennimore indicated no relevant financial relationships.
Debbie Cheng indicated no relevant financial relationships.
Calen Steiner indicated no relevant financial relationships.
Benjamin Click: Jannsen – Consultant. Takeda – Consultant.
Mark Gerich indicated no relevant financial relationships.
Alexis Oonk indicated no relevant financial relationships.
Frank Scott indicated no relevant financial relationships.
John Galatowitsch, MD1, John P. Haydek, MD1, Waseem Ahmed, MD2, Blair Fennimore, MD1, Debbie Cheng, MD1, Calen A. Steiner, MD, MS1, Benjamin Click, MD3, Mark E. Gerich, MD, MBA1, Alexis Oonk, FNP1, Frank I. Scott, MD3. D0385 - Ustekinumab versus Tofacitinib as Second-Line Therapy for Ulcerative Colitis: A Retrospective, Observational Study, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
