Back

Poster Session B - Monday Morning
Category: IBD
B0373 - Corticosteroid Use in Inflammatory Bowel Disease (IBD) Patients May Lead to Worse IBD-Related Outcomes After COVID-19 Infection
Monday, October 24, 2022
10:00 AM – 12:00 PM ET
Location: Crown Ballroom

Has Audio

Adam Spandorfer, MD
Emory University
Atlanta, GA
Presenting Author(s)
Adam Spandorfer, MD, Nicole Lue, MS, Heba Iskandar, MD, Harini Naidu, MD, Lisa Jewell, NP, David Eskreis, MD, Lisa Woolard, PharmD, Meena Prasad, MD, Tanvi Dhere, MD
Emory University, Atlanta, GA
Introduction: There is growing but limited data on the effects COVID-19 has on the disease course of IBD. COVID-19 can enter epithelial cells of the gut via ACE receptors causing cell dysfunction, inflammation, and dysbiosis. Thus, we set out to evaluate IBD outcomes during and three months after COVID-19 infection.
Methods: We performed a retrospective case series comparing IBD patients in remission versus not in remission diagnosed with COVID-19 seen in a single tertiary care center from March 2020 to March 2021. COVID-19 diagnosis was made by positive rapid antigen and/or PCR. We analyzed demographics, medications, need for hospitalization, changes to immunosuppressive therapy, and IBD severity and remission status noted by endoscopic scoring or Physician Global Assessment at the time of COVID-19 diagnosis and 3 months post infection.
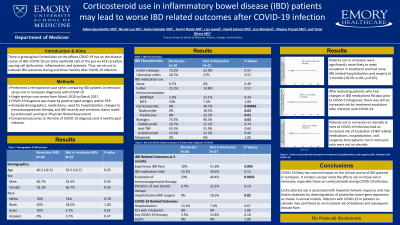
Results: We identified 57 IBD patients, 30 in remission and 27 not in remission, diagnosed with COVID-19. Comparison of baseline characteristics and COVID-19 and IBD related outcomes are noted in Table 1. Patients not in remission were more likely to be on steroids, including prednisone and budesonide, and biologics (0% vs 40.7%, p=0.00001; 73.3% vs 96.3%, p=0.03). Patients not in remission were significantly more likely to need escalation in treatment (OR 15.08; CI 2.98-76.3, p=0.001) and had more IBD related hospitalization and surgery at 3 months compared to patients in remission (18.5% vs 0%, p=0.02). We then excluded patients who had changes in IBD medications 90 days prior to COVID-19 diagnosis and found there was still an increased risk for treatment escalation (OR 7, CI 1.27-38.58, p=0.0254). Additionally, patients not in remission on steroids had an increased risk of escalation of IBD related medications, hospitalization, and surgeries than patients not in remission who were not on steroids (OR 12, CI 1.76-81.7, p=0.0111).
Discussion: Our study suggests COVID-19 likely has minimal impact on the clinical course of IBD patients in remission. It remains unclear what the effects are on those not in remission, especially those on corticosteroids. Corticosteroid use is associated with impaired immune response and may lead to dysbiosis by downregulation of protective mucin gene expression as shown in animal models. Thus, infection with COVID-19 in patients on steroids may contribute to an increased risk of dysbiosis and subsequent disease flare. Further study is warranted to study the effects of steroids on IBD related outcomes in patient with COVID-19 infection.
Disclosures:
Adam Spandorfer, MD, Nicole Lue, MS, Heba Iskandar, MD, Harini Naidu, MD, Lisa Jewell, NP, David Eskreis, MD, Lisa Woolard, PharmD, Meena Prasad, MD, Tanvi Dhere, MD. B0373 - Corticosteroid Use in Inflammatory Bowel Disease (IBD) Patients May Lead to Worse IBD-Related Outcomes After COVID-19 Infection, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
Emory University, Atlanta, GA
Introduction: There is growing but limited data on the effects COVID-19 has on the disease course of IBD. COVID-19 can enter epithelial cells of the gut via ACE receptors causing cell dysfunction, inflammation, and dysbiosis. Thus, we set out to evaluate IBD outcomes during and three months after COVID-19 infection.
Methods: We performed a retrospective case series comparing IBD patients in remission versus not in remission diagnosed with COVID-19 seen in a single tertiary care center from March 2020 to March 2021. COVID-19 diagnosis was made by positive rapid antigen and/or PCR. We analyzed demographics, medications, need for hospitalization, changes to immunosuppressive therapy, and IBD severity and remission status noted by endoscopic scoring or Physician Global Assessment at the time of COVID-19 diagnosis and 3 months post infection.
Results: We identified 57 IBD patients, 30 in remission and 27 not in remission, diagnosed with COVID-19. Comparison of baseline characteristics and COVID-19 and IBD related outcomes are noted in Table 1. Patients not in remission were more likely to be on steroids, including prednisone and budesonide, and biologics (0% vs 40.7%, p=0.00001; 73.3% vs 96.3%, p=0.03). Patients not in remission were significantly more likely to need escalation in treatment (OR 15.08; CI 2.98-76.3, p=0.001) and had more IBD related hospitalization and surgery at 3 months compared to patients in remission (18.5% vs 0%, p=0.02). We then excluded patients who had changes in IBD medications 90 days prior to COVID-19 diagnosis and found there was still an increased risk for treatment escalation (OR 7, CI 1.27-38.58, p=0.0254). Additionally, patients not in remission on steroids had an increased risk of escalation of IBD related medications, hospitalization, and surgeries than patients not in remission who were not on steroids (OR 12, CI 1.76-81.7, p=0.0111).
Discussion: Our study suggests COVID-19 likely has minimal impact on the clinical course of IBD patients in remission. It remains unclear what the effects are on those not in remission, especially those on corticosteroids. Corticosteroid use is associated with impaired immune response and may lead to dysbiosis by downregulation of protective mucin gene expression as shown in animal models. Thus, infection with COVID-19 in patients on steroids may contribute to an increased risk of dysbiosis and subsequent disease flare. Further study is warranted to study the effects of steroids on IBD related outcomes in patient with COVID-19 infection.
| Remission (SD) N = 30 | Not in remission (SD) N = 27 | P-value | |
| Demographics | |||
| Age | 40.2 (16.3) | 35.3 (13.7) | 0.25 |
| Sex | |||
| Male | 46.7% | 33.3% | 0.42 |
| Female | 53.3% | 66.7% | 0.42 |
| Race | |||
| White | 70% | 74% | 0.78 |
| Black | 20% | 18.5% | 1.00 |
| Asian | 10% | 3.7% | 0.61 |
| Hispanic | 0% | 3.7% | 0.47 |
| BMI | 26.2 (5.6) | 24.8 (4.0) | 0.36 |
| Comorbidities | |||
| Organ transplant | 3.3% | 7.4% | 0.60 |
| Cardiovascular disease | 13.3% | 11.1% | 1.00 |
| Diabetes | 3.3% | 3.7% | 1.00 |
| Chronic lung disease | 0% | 3.7% | 0.47 |
| HTN | 10% | 7.4% | 1.00 |
| Current malignancy | 3.3% | 0% | 1.00 |
| CKD | 3.3% | 7.4% | 0.60 |
| Tobacco use | 3.3% | 3.7% | 1.00 |
| Chronic liver disease | 20% | 14.8% | 0.73 |
| IBD Characteristics | |||
| Crohn's disease | 73.3% | 63.0% | 0.57 |
| Ulcerative colitis | 26.7% | 37% | 0.57 |
| Activity Mild Moderate Severe | 14.8% 77.8% 7.4% | ||
| IBD medication use | |||
| None | 6.7% | 0% | 0.49 |
| 5-ASA | 23.3% | 14.8% | 0.51 |
| Immunomodulator | |||
| 6MP/AZA | 3.3% | 11.1% | 0.34 |
| MTX | 10% | 7.4% | 1.00 |
| Corticosteroids | 0% | 40.7% | 0.00001 |
| Budesonide | 0% | 18.5% | 0.02 |
| Prednisone | 0% | 22.2% | 0.01 |
| Biologics | 73.3% | 96.3% | 0.03 |
| Vedolizumab | 16.7% | 22.2% | 0.74 |
| Anti-TNF | 43.3% | 51.9% | 0.60 |
| Ustekinumab | 13.3% | 22.2% | 0.49 |
| Tofacitinib | 0% | 0% | 1.00 |
| COVID-19 Outcomes | |||
| Hospitalization | 13.3% | 7.4% | 0.67 |
| ICU with intubation | 0% | 0% | 1.00 |
| Any COVID-19 therapy | 3.3% | 14.8% | 0.18 |
| Death | 0% | 0% | 1.00 |
| IBD medications held | 13.3% | 29.6% | 0.13 |
| IBD Outcomes at 3 months | |||
| Experienced IBD flare | 10% | 51.8% | 0.005 |
| Escalation of immunosuppression | 10% | 44.4% | 0.00319 |
| Initiation of new steroid therapy | 6.7% | 22.2% | 0.19 |
| IBD related hospitalization/surgery | 0% | 18.5% | 0.02 |
Table: Comparison of IBD related outcomes in patients in remission versus those with active disease at time of COVID-19 diagnosis and 3 months afterwards.
Disclosures:
Adam Spandorfer indicated no relevant financial relationships.
Nicole Lue indicated no relevant financial relationships.
Heba Iskandar indicated no relevant financial relationships.
Harini Naidu indicated no relevant financial relationships.
Lisa Jewell indicated no relevant financial relationships.
David Eskreis indicated no relevant financial relationships.
Lisa Woolard indicated no relevant financial relationships.
Meena Prasad indicated no relevant financial relationships.
Tanvi Dhere indicated no relevant financial relationships.
Adam Spandorfer, MD, Nicole Lue, MS, Heba Iskandar, MD, Harini Naidu, MD, Lisa Jewell, NP, David Eskreis, MD, Lisa Woolard, PharmD, Meena Prasad, MD, Tanvi Dhere, MD. B0373 - Corticosteroid Use in Inflammatory Bowel Disease (IBD) Patients May Lead to Worse IBD-Related Outcomes After COVID-19 Infection, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
