Back

Poster Session E - Tuesday Afternoon
Category: IBD
E0345 - Efficacy of Deucravacitinib, an Oral, Selective, TYK2 Inhibitor, in Patients With Moderately to Severely Active Ulcerative Colitis and Prior Exposure to Biologic Therapy: Subanalysis From the Phase 2 LATTICE-UC Study
Tuesday, October 25, 2022
3:00 PM – 5:00 PM ET
Location: Crown Ballroom

Has Audio

Anita Afzali, MD, MPH, FACG
The Ohio State University Wexner Medical Center
Columbus, Ohio
Presenting Author(s)
Stefan Schreiber, MD1, Nick Powell, MD2, Florian Rieder, MD3, Anita Afzali, MD, MPH, FACG4, Yi Lou, PhD5, Mark Osterman, MD, PhD5, Chun Wu, PhD5, Lisu Wang, MD, MS5, Courtney Radosti, BS5, Aditya Patel, MD, MBA5
1University Hospital Schleswig-Holstein, Kiel University, Kiel, Schleswig-Holstein, Germany; 2Imperial College, London, England, United Kingdom; 3Lerner Research Institute, Cleveland Clinic Foundation, Cleveland, OH; 4The Ohio State University Wexner Medical Center, Columbus, OH; 5Bristol Myers Squibb, Princeton, NJ
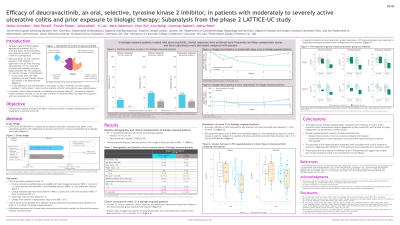
Introduction: Deucravacitinib (DEUC) is an oral, selective, allosteric inhibitor of tyrosine kinase 2 (TYK2), which mediates signaling of key cytokines in UC pathogenesis. In a phase 2 trial of DEUC in moderate to severe active UC, the primary endpoint was not met; however, a treatment effect was observed in patients with prior exposure to ≥1 biologic agent.
Methods: LATTICE-UC (NCT03934216), a double-blind phase 2 trial, randomized patients with moderate to severe active UC (modified Mayo score [MMS] 5-9 with endoscopic score [MES] ≥2, rectal bleeding score [RBS] ≥1, stool frequency score [SFS] ≥2) 2:1 to DEUC 6 mg or placebo (PBO) twice daily (BID). This post-hoc analysis in biologic-exposed patients assessed clinical remission (MMS ≤2, with SFS ≤1, RBS=0, MES ≤1); clinical response (decrease from baseline [BL] in MMS ≥2 points and ≥30% with decrease in RBS ≥1 point or absolute RBS ≤1); endoscopic improvement (MES ≤1); and change from BL (CFB) in symptomatic Mayo score (RBS + SFS). Colonic tissue transcriptomes were assessed via bulk RNA sequencing in a subset of biologic-exposed patients (DEUC n=17; PBO n=9). Differential expression with limma-voom and pathway enrichment analysis via Gene Set Enrichment Analysis were performed.
Results: Of 131 patients randomized, 48 (36.6%) were biologic-exposed (DEUC, 32/88 [36.4%]; PBO, 16/43 [37.2%]). At week 12, higher response rates were seen in patients receiving DEUC vs PBO in clinical remission (16.1% vs 0.0%), clinical response (29.0% vs 12.5%), and endoscopic improvement (25.8% vs 12.5%). Greater mean CFB in symptomatic Mayo score was observed at week 12 with DEUC (-2.1) vs PBO (-0.1) (Table). Type I interferon (IFN)‒regulated genes (IRG) were significantly reduced in colonic tissues at week 12 compared to BL in DEUC (FDR < 0.1) but not in PBO. Pathway enrichment analysis confirmed that the IFN pathway was down-regulated with DEUC treatment. In patients receiving DEUC who achieved clinical response at week 12, IRG expression was reduced compared with nonresponders; similar trends were seen in clinical remitters and in endoscopic improvers.
Discussion: Biologic-exposed patients treated with DEUC had greater improvements in clinical outcomes compared to PBO and had greater decreases in colonic IRG. The biomarker decreases were associated with clinical response or remission, suggesting inhibition of TYK2 pathways may be beneficial for UC. These results provide evidence the target was engaged and suggest a higher dose may have greater efficacy.
Disclosures:
Stefan Schreiber, MD1, Nick Powell, MD2, Florian Rieder, MD3, Anita Afzali, MD, MPH, FACG4, Yi Lou, PhD5, Mark Osterman, MD, PhD5, Chun Wu, PhD5, Lisu Wang, MD, MS5, Courtney Radosti, BS5, Aditya Patel, MD, MBA5. E0345 - Efficacy of Deucravacitinib, an Oral, Selective, TYK2 Inhibitor, in Patients With Moderately to Severely Active Ulcerative Colitis and Prior Exposure to Biologic Therapy: Subanalysis From the Phase 2 LATTICE-UC Study, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
1University Hospital Schleswig-Holstein, Kiel University, Kiel, Schleswig-Holstein, Germany; 2Imperial College, London, England, United Kingdom; 3Lerner Research Institute, Cleveland Clinic Foundation, Cleveland, OH; 4The Ohio State University Wexner Medical Center, Columbus, OH; 5Bristol Myers Squibb, Princeton, NJ
Introduction: Deucravacitinib (DEUC) is an oral, selective, allosteric inhibitor of tyrosine kinase 2 (TYK2), which mediates signaling of key cytokines in UC pathogenesis. In a phase 2 trial of DEUC in moderate to severe active UC, the primary endpoint was not met; however, a treatment effect was observed in patients with prior exposure to ≥1 biologic agent.
Methods: LATTICE-UC (NCT03934216), a double-blind phase 2 trial, randomized patients with moderate to severe active UC (modified Mayo score [MMS] 5-9 with endoscopic score [MES] ≥2, rectal bleeding score [RBS] ≥1, stool frequency score [SFS] ≥2) 2:1 to DEUC 6 mg or placebo (PBO) twice daily (BID). This post-hoc analysis in biologic-exposed patients assessed clinical remission (MMS ≤2, with SFS ≤1, RBS=0, MES ≤1); clinical response (decrease from baseline [BL] in MMS ≥2 points and ≥30% with decrease in RBS ≥1 point or absolute RBS ≤1); endoscopic improvement (MES ≤1); and change from BL (CFB) in symptomatic Mayo score (RBS + SFS). Colonic tissue transcriptomes were assessed via bulk RNA sequencing in a subset of biologic-exposed patients (DEUC n=17; PBO n=9). Differential expression with limma-voom and pathway enrichment analysis via Gene Set Enrichment Analysis were performed.
Results: Of 131 patients randomized, 48 (36.6%) were biologic-exposed (DEUC, 32/88 [36.4%]; PBO, 16/43 [37.2%]). At week 12, higher response rates were seen in patients receiving DEUC vs PBO in clinical remission (16.1% vs 0.0%), clinical response (29.0% vs 12.5%), and endoscopic improvement (25.8% vs 12.5%). Greater mean CFB in symptomatic Mayo score was observed at week 12 with DEUC (-2.1) vs PBO (-0.1) (Table). Type I interferon (IFN)‒regulated genes (IRG) were significantly reduced in colonic tissues at week 12 compared to BL in DEUC (FDR < 0.1) but not in PBO. Pathway enrichment analysis confirmed that the IFN pathway was down-regulated with DEUC treatment. In patients receiving DEUC who achieved clinical response at week 12, IRG expression was reduced compared with nonresponders; similar trends were seen in clinical remitters and in endoscopic improvers.
Discussion: Biologic-exposed patients treated with DEUC had greater improvements in clinical outcomes compared to PBO and had greater decreases in colonic IRG. The biomarker decreases were associated with clinical response or remission, suggesting inhibition of TYK2 pathways may be beneficial for UC. These results provide evidence the target was engaged and suggest a higher dose may have greater efficacy.
Table. Clinical Endpoints at Week 12 in Bio-Exposed Patients
| Endpoint | Deucravacitinib 6 mg BID (n = 31) | Placebo (n = 16) |
| Clinical remission response rate, mean | 16.1% | 0.0% |
| Clinical response rate, mean | 29.0% | 12.5% |
| Endoscopic improvement response rate, mean | 25.8% | 12.5% |
| Symptomatic Mayo score, mean change from baseline (a) | -2.1% | -0.1 |
Table: (a) n for deucravacitinib and placebo is 16 and 9, respectively. BID, twice daily.
Disclosures:
Stefan Schreiber: AbbVie – Consultant, Personal fees. Arena – Consultant, Personal fees. Biogen – Consultant, Personal fees. Bristol-Myers Squibb – Consultant, Personal fees. Celgene – Consultant, Personal fees. Celltrion – Consultant, Personal fees. Eli Lilly and Company – Consultant, Personal fees. Falk – Consultant, Personal fees. Ferring – Consultant, Personal fees. Fresenius – Consultant, Personal fees. Galapagos/Gilead Sciences – Consultant, Personal fees. IMAB – Consultant, Personal fees. Janssen – Consultant, Personal fees. MSD – Consultant, Personal fees. Mylan – Consultant, Personal fees. Pfizer Inc – Consultant, Personal fees. Protagonist – Consultant, Personal fees. Provention Bio – Consultant, Personal fees. Takeda – Consultant, Personal fees. Theravance – Consultant, Personal fees.
Nick Powell: AbbVie – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Allergan – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. AstraZeneca – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Celgene – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Celltrion – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Dr Falk Pharma UK Ltd – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Ferring – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Galapagos – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Janssen – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Pfizer – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Roche – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Sobi – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Takeda – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Tillotts – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Vifor – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau.
Florian Rieder: 89Bio – Advisory Committee/Board Member, Consultant. AbbVie – Advisory Committee/Board Member, Consultant. Adnovate – Advisory Committee/Board Member, Consultant. Agomab – Advisory Committee/Board Member, Consultant. Allergan – Advisory Committee/Board Member, Consultant. Arena – Advisory Committee/Board Member, Consultant. Boehringer-Ingelheim – Advisory Committee/Board Member, Consultant. CDISC – Advisory Committee/Board Member, Consultant. Celgene/BMS – Advisory Committee/Board Member, Consultant. Cowen – Advisory Committee/Board Member, Consultant. Ferring – Advisory Committee/Board Member, Consultant. Galapagos – Advisory Committee/Board Member, Consultant. Galmed – Advisory Committee/Board Member, Consultant. Genentech – Advisory Committee/Board Member, Consultant. Gilead – Advisory Committee/Board Member, Consultant. Gossamer – Advisory Committee/Board Member, Consultant. Guidepoint – Advisory Committee/Board Member, Consultant. Helmsley – Advisory Committee/Board Member, Consultant. Horizon Therapeutics – Advisory Committee/Board Member, Consultant. Image Analysis Limited – Advisory Committee/Board Member, Consultant. Index Pharma – Advisory Committee/Board Member, Consultant. Janssen – Advisory Committee/Board Member, Consultant. Koutif – Advisory Committee/Board Member, Consultant. Mestag – Advisory Committee/Board Member, Consultant. Metacrine – Advisory Committee/Board Member, Consultant. Morphic – Advisory Committee/Board Member, Consultant. Organovo – Advisory Committee/Board Member, Consultant. Origo – Advisory Committee/Board Member, Consultant. Pfizer – Advisory Committee/Board Member, Consultant. Pliant – Advisory Committee/Board Member, Consultant. Prometheus Biosciences – Advisory Committee/Board Member, Consultant. Receptos – Advisory Committee/Board Member, Consultant. RedX – Advisory Committee/Board Member, Consultant. Roche – Advisory Committee/Board Member, Consultant. Samsung – Advisory Committee/Board Member, Consultant. Surmodics – Advisory Committee/Board Member, Consultant. Surrozen – Advisory Committee/Board Member, Consultant. Takeda – Advisory Committee/Board Member, Consultant. Techlab – Advisory Committee/Board Member, Consultant. Theravance – Advisory Committee/Board Member, Consultant. Thetis – Advisory Committee/Board Member, Consultant. UCB – Advisory Committee/Board Member, Consultant. Ysios – Advisory Committee/Board Member, Consultant.
Anita Afzali: AbbVie – Consultant, Speakers Bureau. Bristol Myers Squibb – Consultant, Speakers Bureau. DiaSorin – Consultant. Eli Lilly – Consultant. Gilead – Consultant. Janssen – Consultant, Speakers Bureau. Pfizer – Consultant, Speakers Bureau. Takeda – Consultant, Speakers Bureau. TLL Pharmaceuticals – Consultant.
Yi Lou: Bristol Myers Squibb – Employee, Stock-publicly held company(excluding mutual/index funds).
Mark Osterman: Bristol Myers Squibb – Employee, Stock-publicly held company(excluding mutual/index funds).
Chun Wu: Bristol Myers Squibb – Employee, Stock-publicly held company(excluding mutual/index funds).
Lisu Wang: Bristol Myers Squibb – Employee, Stock-publicly held company(excluding mutual/index funds).
Courtney Radosti: Bristol Myers Squibb – Employee, Stock-publicly held company(excluding mutual/index funds).
Aditya Patel: Bristol Myers Squibb – Employee, Stock-publicly held company(excluding mutual/index funds).
Stefan Schreiber, MD1, Nick Powell, MD2, Florian Rieder, MD3, Anita Afzali, MD, MPH, FACG4, Yi Lou, PhD5, Mark Osterman, MD, PhD5, Chun Wu, PhD5, Lisu Wang, MD, MS5, Courtney Radosti, BS5, Aditya Patel, MD, MBA5. E0345 - Efficacy of Deucravacitinib, an Oral, Selective, TYK2 Inhibitor, in Patients With Moderately to Severely Active Ulcerative Colitis and Prior Exposure to Biologic Therapy: Subanalysis From the Phase 2 LATTICE-UC Study, ACG 2022 Annual Scientific Meeting Abstracts. Charlotte, NC: American College of Gastroenterology.
